OCR Specification focus:
‘Recall approximate wavelength orders from radio to gamma rays.’
Electromagnetic (EM) waves span an immense range of wavelengths, from kilometres in radio waves to less than a picometre in gamma rays. Understanding these orders of magnitude helps classify EM waves, appreciate their varied applications, and predict their interactions with matter.
The Electromagnetic Spectrum Overview
The electromagnetic spectrum includes all possible frequencies of electromagnetic radiation. Each region corresponds to a range of wavelengths and frequencies but shares the same fundamental wave nature — oscillating electric and magnetic fields that propagate through space at the speed of light in a vacuum (c ≈ 3.00 × 10⁸ m s⁻¹).
Despite differing enormously in scale, all EM waves exhibit the same basic behaviour, including reflection, refraction, and diffraction. Their classification depends solely on wavelength (λ) or frequency (f), linked by the fundamental relationship:
EQUATION
—-----------------------------------------------------------------
Wave Speed Equation (v = fλ)
v = wave speed (m s⁻¹)
f = frequency (Hz)
λ = wavelength (m)
—-----------------------------------------------------------------
In a vacuum, v = c, so longer wavelengths correspond to lower frequencies and vice versa.
Orders of Magnitude across the Spectrum
To “recall approximate wavelength orders” means to know the typical powers of ten that describe the wavelength ranges of each EM band. The spectrum can be ordered from the longest to the shortest wavelengths as follows:
Radio waves: 10³ to 10⁻¹ m
Microwaves: 10⁻¹ to 10⁻³ m
Infrared (IR): 10⁻³ to 7 × 10⁻⁷ m
Visible light: 7 × 10⁻⁷ to 4 × 10⁻⁷ m
Ultraviolet (UV): 4 × 10⁻⁷ to 10⁻⁸ m
X-rays: 10⁻⁸ to 10⁻¹¹ m
Gamma rays: <10⁻¹¹ m
These are approximate, overlapping boundaries — real sources emit a continuous range of wavelengths rather than distinct categories.
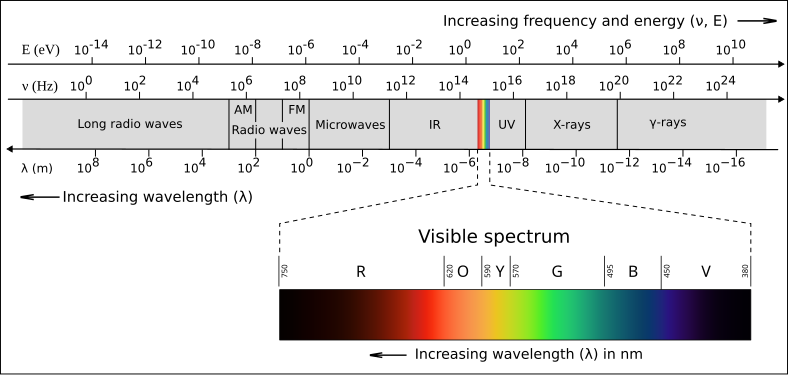
A clean diagram of the electromagnetic spectrum from radio to gamma rays, labelled by wavelength (m), frequency (Hz), and photon energy (eV). The powers-of-ten scale highlights how wavelength decreases by orders of magnitude across the spectrum. This image includes an energy axis in addition to wavelength/frequency; that extra detail is useful context but not required by the syllabus. Source.
Radio Waves (λ ≈ 10³ m to 10⁻¹ m)
Radio waves have the longest wavelengths and lowest frequencies in the spectrum.
They are produced by oscillating electrical currents in antennas and are used for broadcasting, communication, and radar.
Because of their long wavelength, they can diffract around obstacles and reflect off the ionosphere, allowing long-distance transmission.
Microwaves (λ ≈ 10⁻¹ m to 10⁻³ m)
Microwaves are shorter than radio waves but still relatively long compared with visible light.
They are used in satellite communication, microwave ovens, and Doppler radar systems. Their wavelengths correspond to sizes comparable to water molecules, making them effective for heating food by causing molecular rotation.
Infrared Radiation (λ ≈ 10⁻³ m to 7 × 10⁻⁷ m)
Infrared waves are emitted by warm objects, corresponding to the thermal radiation humans can sense as heat.
Applications include night-vision cameras, remote controls, and thermal imaging.
Their wavelengths are slightly longer than visible red light, allowing efficient energy transfer without ionisation.
Visible Light (λ ≈ 7 × 10⁻⁷ m to 4 × 10⁻⁷ m)
This is the narrow band detectable by the human eye.
The visible spectrum runs from red (longest wavelength) to violet (shortest wavelength).

A linear bar of the visible spectrum labelled in nanometres, showing the continuous transition from approximately 700 nm (red) to 400 nm (violet). This focuses purely on the visible range to reinforce the typical 10⁻⁷ m order of magnitude. The diagram is intentionally minimal, avoiding applications or unrelated details. Source.
Though it spans less than a factor of two in wavelength, this region contains all perceived colours and is essential to human vision and photosynthesis.
Ultraviolet Radiation (λ ≈ 4 × 10⁻⁷ m to 10⁻⁸ m)
Ultraviolet (UV) lies beyond violet light and possesses higher frequencies and energies.
It can cause ionisation in atoms and molecules, making it biologically hazardous in excess.
However, it is useful for sterilisation, fluorescent lighting, and detecting forged banknotes.
The atmosphere partially absorbs UV radiation, especially by the ozone layer.
X-Rays (λ ≈ 10⁻⁸ m to 10⁻¹¹ m)
X-rays have much shorter wavelengths, giving them high photon energies.
They are produced when high-speed electrons strike metal targets, leading to medical imaging and crystal diffraction studies.
Because they can penetrate soft tissue but are absorbed by bone, they reveal internal structures clearly.
Gamma Rays (λ < 10⁻¹¹ m)
Gamma rays are the shortest-wavelength and highest-energy electromagnetic waves.
They originate from nuclear decay, cosmic phenomena, and particle interactions.
Their extreme energy allows deep penetration through matter, useful in cancer radiotherapy but also potentially dangerous to living cells.
Relationship Between Wavelength and Energy
As wavelength decreases across the EM spectrum, frequency and therefore photon energy increase. The relationship is given by:
EQUATION
—-----------------------------------------------------------------
Photon Energy (E = hf)
E = photon energy (J)
h = Planck’s constant (6.63 × 10⁻³⁴ J s)
f = frequency (Hz)
—-----------------------------------------------------------------
This explains why gamma rays and X-rays are ionising — their photons carry enough energy to remove electrons from atoms. Conversely, radio waves are non-ionising, interacting primarily through oscillations of electric charges.
Scientific Use of Orders of Magnitude
Physicists often use orders of magnitude (powers of ten) to express large or small quantities efficiently. For EM waves, this helps:
Estimate energy levels: A tenfold decrease in wavelength corresponds to a tenfold increase in frequency.
Predict interactions: Long wavelengths interact with macroscopic objects (antennas), while short wavelengths affect atomic or subatomic scales.
Design experiments: Knowing wavelength orders determines appropriate apparatus — for example, diffraction gratings for visible light or crystal lattices for X-rays.
Logarithmic Representation
Because the EM spectrum spans about 20 orders of magnitude, logarithmic scales are often used to visualise it.
Each order of magnitude step corresponds to multiplying or dividing wavelength by ten.
For example:
Radio (10⁰ m) → Microwave (10⁻² m) → Infrared (10⁻⁴ m) → Visible (10⁻⁶ m) → UV (10⁻⁷ m) → X-ray (10⁻¹⁰ m) → Gamma (10⁻¹² m).
This continuous progression illustrates the unified nature of electromagnetic radiation despite its diversity in practical applications.
Applications Linked to Wavelength Orders
Communication: Radio and microwaves transmit data via amplitude or frequency modulation.
Imaging: Infrared and visible light enable optical instruments and thermal cameras.
Medical and scientific use: X-rays and gamma rays probe atomic structures and treat diseases.
Environmental monitoring: UV and IR sensors analyse atmospheric composition and climate effects.
Each application exploits the specific interaction between wavelength and matter, showing how understanding the orders of magnitude guides technological development.
FAQ
Because the electromagnetic spectrum covers an enormous range of wavelengths—from kilometres to less than a trillionth of a metre—expressing them as powers of ten allows quick comparison across many scales.
For instance, visible light at 10⁻⁷ m and radio waves at 10³ m differ by ten orders of magnitude. Writing values in scientific notation makes it easy to see these proportional relationships and simplifies calculations involving frequency and energy.
Such short wavelengths cannot be measured directly using ordinary rulers or wave interference methods. Instead, scientists use indirect techniques such as:
Diffraction patterns produced when X-rays pass through crystal lattices, allowing calculation of wavelength using spacing between atomic planes.
Energy–frequency conversion, applying the relationship E = hf to determine wavelength from photon energy.
These approaches are crucial for studying atomic and nuclear-scale phenomena.
The divisions (e.g., between infrared and visible light) are human-defined categories based on source, detection method, and typical application rather than sharp physical transitions.
In reality, electromagnetic radiation forms a continuous spectrum. The overlap occurs because many sources emit a broad range of wavelengths, and detection technologies may respond across multiple adjacent bands.
Ionisation depends on photon energy, which is linked to frequency.
High-frequency radiation (ultraviolet, X-rays, gamma rays) has photons energetic enough to remove electrons from atoms or molecules, creating ions.
Low-frequency radiation (radio, microwave, infrared) transfers energy more gradually, causing heating or molecular vibrations rather than ionisation.
This distinction is essential for assessing biological and safety effects of radiation exposure.
While frequency remains constant when light passes from one medium to another, wavelength changes because wave speed changes.
In denser media (e.g., glass), light travels slower, so wavelength becomes shorter.
In less dense media (e.g., air or vacuum), speed increases, so wavelength lengthens.
This variation is responsible for refraction—the bending of light at material boundaries—and is described quantitatively by the refractive index (n = c/v).
Practice Questions
Question 1 (2 marks)
State the approximate wavelength ranges of (a) radio waves and (b) visible light in the electromagnetic spectrum.
Mark scheme:
(a) Radio waves: wavelength range approximately 10³ m to 10⁻¹ m (1 mark)
(b) Visible light: wavelength range approximately 7 × 10⁻⁷ m to 4 × 10⁻⁷ m (1 mark)
Question 2 (5 marks)
The electromagnetic spectrum covers a wide range of wavelengths and frequencies.
(a) Explain what is meant by an order of magnitude in the context of the electromagnetic spectrum.
(b) Describe how the energy and frequency of electromagnetic waves change as wavelength decreases across the spectrum, from radio waves to gamma rays.
(c) Explain why gamma rays are more hazardous to living tissue than radio waves.
Mark scheme:
(a)
Definition: An order of magnitude refers to a tenfold (factor of 10) change in size or value (1 mark).
Used to compare wavelength ranges or scales across the spectrum (1 mark).
(b)
As wavelength decreases, frequency increases (1 mark).
Photon energy increases because energy is proportional to frequency (E = hf) (1 mark).
(c)
Gamma rays are ionising; their photons carry enough energy to remove electrons from atoms or molecules (1 mark).
Radio waves are non-ionising and mainly cause oscillations of charged particles rather than ionisation (1 mark).

