OCR Specification focus:
‘Define specific latent heats of fusion and vaporisation; relate energy by E = mL.’
Specific latent heat explains how energy is absorbed or released during phase changes of a substance without a change in temperature. It’s vital for understanding melting, boiling, and energy transfer processes in thermal physics.
Specific Latent Heat and Phase Change
When a substance changes state — such as from solid to liquid (melting) or liquid to gas (boiling) — energy must be supplied to overcome the intermolecular forces binding the particles together. During this phase change, the temperature remains constant, even though energy is being transferred. This absorbed or released energy is known as latent heat.
Specific latent heat: The amount of energy required to change the state of 1 kilogram of a substance without any change in temperature.
The term latent means “hidden,” referring to the fact that this energy is not observable as a temperature rise but is stored as potential energy within the structure of the material.
Phase Changes and Molecular Behaviour
The absorption or release of latent heat alters the potential energy of the particles:
Melting (fusion): Energy is absorbed to weaken or break the bonds holding molecules in a solid lattice. The particles gain potential energy but retain the same average kinetic energy, so temperature stays constant.
Boiling (vaporisation): Energy is absorbed to completely separate molecules so they can move freely as a gas. The potential energy increases substantially while the temperature remains constant until all liquid becomes gas.
Freezing and condensation: The reverse processes release latent heat as molecules form stronger intermolecular bonds.
Each substance has characteristic values for these energy transfers — its specific latent heats — which depend on its molecular structure and strength of intermolecular forces.
Types of Specific Latent Heat
There are two key types recognised in the OCR specification:
Specific Latent Heat of Fusion (Lf)
This applies to the solid–liquid transition. It is the energy needed per kilogram to convert a solid into a liquid at constant temperature, or released when a liquid freezes.
Specific Latent Heat of Vaporisation (Lv)
This refers to the liquid–gas transition. It is the energy needed per kilogram to convert a liquid to a gas at constant temperature, or released when a gas condenses.
These two quantities are distinct because intermolecular forces in solids and liquids differ significantly. Liquids require more energy to vaporise than solids need to melt, since molecules must be fully separated to become gas.
The Energy–Mass Relationship
The relationship between energy, mass, and specific latent heat is fundamental to the study of thermal energy transfer in phase changes.
EQUATION
—-----------------------------------------------------------------
Energy of phase change (E) = mL
E = Energy required or released (joules, J)
m = Mass of the substance (kilograms, kg)
L = Specific latent heat (joules per kilogram, J kg⁻¹)
—-----------------------------------------------------------------
This formula quantifies how much energy is involved in changing the state of a known mass of a material. For example, doubling the mass doubles the energy needed, since more particles require energy to overcome their bonds.
After applying the energy, the system’s temperature does not rise until the entire sample has completed its phase change. This illustrates the distinction between specific heat capacity (which relates to temperature change) and specific latent heat (which relates to state change).
Microscopic Interpretation
At the molecular level, when a solid melts or a liquid boils, the kinetic energy of particles remains roughly constant, because temperature — a measure of average kinetic energy — does not change.
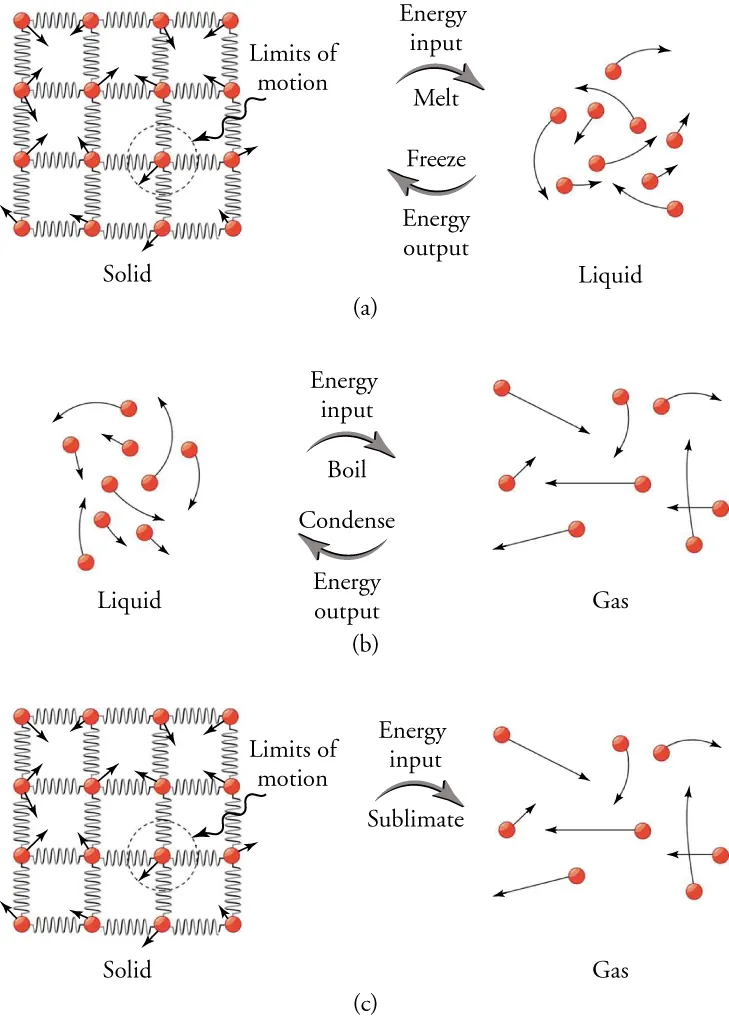
Phase-change energetics at the particle level. Energy input weakens or breaks intermolecular attractions during melting and vaporisation; energy is released when bonds form during freezing and condensation. Temperature remains unchanged until the phase change completes (extra labels for sublimation/condensation appear but are consistent with the same principle). Source.
Instead, potential energy increases as intermolecular bonds are broken or weakened.
During melting, molecules vibrate more freely as they escape fixed lattice positions.
During boiling, molecules completely overcome cohesive forces, moving apart to form a gas.
In freezing or condensation, the potential energy decreases as bonds form, releasing latent heat into the surroundings.
This conversion between potential and thermal energy underpins many natural and engineered thermal processes.
Factors Affecting Specific Latent Heat
The value of specific latent heat depends on:
Type of substance: Materials with strong intermolecular forces (e.g. water) require more energy for phase change.
Phase change type: Latent heat of vaporisation is typically much larger than that of fusion because separating particles into a gas requires more energy.
Pressure: Changing pressure alters the melting and boiling points, slightly affecting latent heat values.
Purity of material: Impurities disrupt regular bonding patterns, reducing the energy needed for phase changes.
Energy Transfer and Practical Significance
Latent heat processes play a major role in both everyday phenomena and technological applications. Understanding them helps explain and control how energy is transferred during state changes.
Examples include:
Refrigeration and air conditioning: These rely on substances evaporating and condensing to absorb and release latent heat, maintaining temperature control.
Meteorology: Evaporation and condensation of water influence cloud formation and energy exchange in the atmosphere.
Thermal management systems: Phase-change materials use latent heat storage to regulate temperature in buildings or electronics.
In each case, latent heat allows large energy transfers to occur at constant temperature, a critical principle for energy efficiency and thermal control.
Graphical Representation of Phase Changes
When plotting temperature against energy supplied, the graph shows horizontal regions where temperature remains constant.
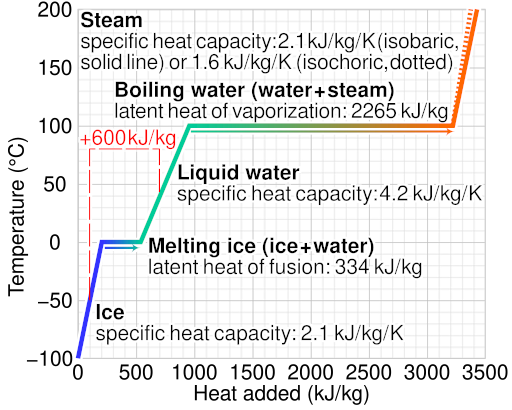
Heating curve for water showing temperature as a function of energy supplied at constant pressure. The plateaus at 0 °C and 100 °C correspond to latent heats of fusion and vaporisation, respectively. During each plateau, energy input increases potential energy while temperature remains constant. Source.
These plateaus correspond to phase changes — during melting or boiling — where latent heat is absorbed without a rise in temperature.
The steeper slopes between plateaus represent heating within a single phase, governed by specific heat capacity rather than latent heat. Recognising these regions is key to interpreting experimental or real-world data.
Summary of Conceptual Relationships
While specific heat capacity measures the energy required to raise the temperature of a substance, specific latent heat measures the energy for a change of state at constant temperature. Together, they provide a complete description of how energy affects matter thermally.
Understanding the equation E = mL allows students to calculate the energy involved in melting, boiling, or condensing a substance, linking macroscopic energy transfer to microscopic particle behaviour and the principles of thermodynamics.
FAQ
Specific latent heat depends mainly on the strength and type of intermolecular forces between particles.
Substances with strong hydrogen bonding, like water, have high latent heats because more energy is required to separate molecules.
Substances with weaker Van der Waals forces, like noble gases, have low latent heats since little energy is needed to overcome attractions.
Atomic mass and structure also play roles — heavier molecules often require more energy to move apart due to stronger London dispersion forces.
During a phase change, the supplied energy increases the potential energy of the particles rather than their kinetic energy.
Since temperature measures average kinetic energy, there is no temperature rise.
The energy instead goes into weakening or breaking intermolecular bonds until the entire substance has changed state. Only after the phase change is complete does further energy raise the temperature.
Yes, specific latent heat varies slightly with pressure because changing pressure alters the melting or boiling point.
At higher pressures:
Molecules are closer together, so more energy is required to separate them, slightly increasing latent heat.
At lower pressures:The substance boils at a lower temperature and requires less energy per kilogram for vaporisation.
This effect is small under normal atmospheric conditions but significant in high-pressure or vacuum systems.
It can be determined using an electrical heating method where the energy supplied is measured as current × voltage × time.
For vaporisation:
A known mass of liquid is boiled using an immersion heater.
Energy supplied is recorded, and mass loss due to evaporation is measured.
Specific latent heat is found from E = mL.
For fusion:A solid is melted using electrical heating while ensuring temperature remains constant throughout melting.
During fusion, only some intermolecular bonds are broken as the solid turns into a liquid, where molecules remain relatively close.
During vaporisation, molecules must completely overcome all attractive forces to separate into a gas.
This requires a much larger energy input per kilogram, leading to significantly higher specific latent heat values for vaporisation compared to fusion.
For example, water’s latent heat of vaporisation (≈2.26 × 10⁶ J kg⁻¹) is more than six times greater than its latent heat of fusion (≈3.34 × 10⁵ J kg⁻¹).
Practice Questions
Question 1 (2 marks)
Define specific latent heat of vaporisation and explain what happens to the energy supplied to a liquid during vaporisation.
Mark scheme
1 mark: Correctly states that specific latent heat of vaporisation is the energy required to change 1 kg of a substance from liquid to gas without a change in temperature.
1 mark: Explains that the energy supplied increases the potential energy of the particles by breaking intermolecular forces, while the temperature (and hence kinetic energy) remains constant.
Question 2 (5 marks)
Water at 0 °C is heated until it completely changes from ice to steam at 100 °C.
Explain, in terms of energy transfer and molecular behaviour, what happens to the energy supplied to the system at each stage of this process.
Mark scheme
1 mark: States that energy first increases the kinetic energy of particles in the ice, raising its temperature until 0 °C.
1 mark: Recognises that during melting (fusion), temperature remains constant while energy is used to break bonds between particles (latent heat of fusion).
1 mark: Explains that once melted, further heating increases kinetic energy, raising the liquid’s temperature to 100 °C.
1 mark: States that during boiling (vaporisation), temperature remains constant while energy is used to fully separate particles (latent heat of vaporisation).
1 mark: Notes that in the gaseous state, any additional energy supplied increases the particles’ kinetic energy and thus the gas temperature.

