OCR Specification focus:
‘With pV = NkT and ½m⟨c²⟩ = 3/2 kT, determine the internal energy of an ideal gas.’
In an ideal gas, the microscopic behaviour of molecules determines its macroscopic properties. Understanding the Boltzmann constant and internal energy links the motion of individual particles to the overall energy and temperature of the system.
The Boltzmann Constant and Molecular Energy
The Boltzmann constant (k) is a fundamental physical constant that connects the average kinetic energy of particles in a gas to the absolute temperature (T). It forms a bridge between the microscopic and macroscopic worlds in thermodynamics.
Boltzmann Constant (k): The proportionality constant relating the mean kinetic energy of particles in a gas to its absolute temperature.
This constant allows temperature, a macroscopic quantity, to be expressed in terms of microscopic energy. It provides the link between the ideal gas equation written for moles and the same equation expressed in terms of individual particles.
The Ideal Gas Equation in Terms of Molecules
The macroscopic ideal gas equation is given by pV = nRT, where R is the molar gas constant. When considering individual molecules rather than moles, the equation can be rewritten using the Boltzmann constant:
EQUATION
—-----------------------------------------------------------------
Equation of State for an Ideal Gas (molecular form): pV = NkT
p = Pressure (Pa)
V = Volume (m³)
N = Number of molecules
k = Boltzmann constant (1.38 × 10⁻²³ J K⁻¹)
T = Absolute temperature (K)
—-----------------------------------------------------------------
This expression indicates that the pressure, volume, and temperature of an ideal gas are determined by the behaviour of its constituent molecules. Each molecule contributes to the total pressure through collisions with the container walls, and these collisions depend on the kinetic energy of the particles.
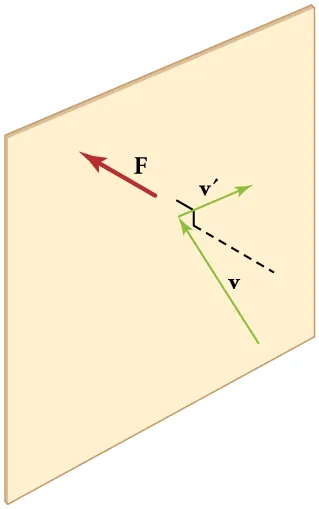
Elastic collision of a molecule with a wall reverses the perpendicular velocity component, producing an impulse and hence a force. The average of many such collisions generates gas pressure — the microscopic origin of the macroscopic pressure term in the gas laws. Source.
Molecular Kinetic Energy and Temperature
The motion of gas molecules is random and continuous. The kinetic theory of gases provides a link between molecular motion and temperature. For a monatomic ideal gas, the mean kinetic energy of a single molecule is proportional to the absolute temperature.
EQUATION
—-----------------------------------------------------------------
Mean Kinetic Energy of a Molecule: ½m⟨c²⟩ = 3/2 kT
m = Mass of one molecule (kg)
⟨c²⟩ = Mean square speed of molecules (m² s⁻²)
k = Boltzmann constant (J K⁻¹)
T = Absolute temperature (K)
—-----------------------------------------------------------------
This equation demonstrates that as the temperature increases, the average speed and hence kinetic energy of the molecules also increase. The root mean square (r.m.s.) speed, denoted by crms = √⟨c²⟩, is a useful measure of molecular motion and depends only on temperature and molecular mass.
Between any two given temperatures, the ratio of molecular speeds can be compared through the square root of their temperature ratio. This directly connects microscopic kinetic energy with the macroscopic concept of temperature.
Internal Energy of an Ideal Gas
The internal energy (U) of a system refers to the total microscopic energy contained within it due to both kinetic and potential energies of its molecules.
Internal Energy (U): The sum of the random kinetic energies and potential energies of all molecules in a system.
For an ideal gas, the assumption is that there are no intermolecular forces and thus no potential energy between molecules. Consequently, the internal energy depends entirely on the random kinetic energy of the molecules.
EQUATION
—-----------------------------------------------------------------
Internal Energy of an Ideal Gas: U = (3/2) NkT
U = Internal energy (J)
N = Number of molecules
k = Boltzmann constant (J K⁻¹)
T = Absolute temperature (K)
—-----------------------------------------------------------------
Alternatively, since Nk = nR, the same expression can be written as U = (3/2) nRT for macroscopic systems expressed in moles. This shows that internal energy depends only on temperature and the amount of substance.
Increases in internal energy correspond directly to increases in temperature, as additional energy manifests as faster molecular motion rather than potential energy changes.
Physical Interpretation
Each degree of freedom of a molecule contributes equally to its internal energy, according to the equipartition of energy principle.
For a monatomic gas (e.g. helium, neon), there are three translational degrees of freedom — one for each spatial dimension.
Each degree of freedom contributes ½kT to the energy per molecule.
Therefore, total kinetic energy per molecule = 3 × ½kT = 3/2 kT.
For a collection of N molecules, this scales to U = (3/2) NkT, matching the earlier derived result.
In polyatomic gases, rotational and vibrational degrees of freedom add extra contributions to internal energy. However, for the purposes of the OCR A-Level Physics specification, only monatomic ideal gases are considered in this derivation.
Relationship Between pV and Internal Energy
Combining the molecular form of the ideal gas law (pV = NkT) with the kinetic energy relation (½m⟨c²⟩ = 3/2 kT) gives:
EQUATION
—-----------------------------------------------------------------
Microscopic–Macroscopic Relation: pV = (1/3) Nm⟨c²⟩
p = Pressure (Pa)
V = Volume (m³)
N = Number of molecules
m = Mass of one molecule (kg)
⟨c²⟩ = Mean square speed (m² s⁻²)
—-----------------------------------------------------------------
This equation links the macroscopic measurable quantities (p and V) to the microscopic behaviour of gas molecules (m⟨c²⟩). It confirms that gas pressure arises from molecular motion and that the magnitude of internal energy is determined by these random kinetic contributions.
A cubic container of gas with molecular speeds resolved into x, y, z components. Averaging the squared components gives ⟨vx2⟩=⟨vy2⟩=⟨vz2⟩=13⟨v2⟩\langle v_x^2\rangle=\langle v_y^2\rangle=\langle v_z^2\rangle=\tfrac{1}{3}\langle v^2\rangle⟨vx2⟩=⟨vy2⟩=⟨vz2⟩=31⟨v2⟩, leading to pV=13Nm⟨v2⟩pV=\tfrac{1}{3}Nm\langle v^2\ranglepV=31Nm⟨v2⟩. This connects molecular motion to macroscopic pressure and underpins the internal energy relation U=32NkTU=\tfrac{3}{2}NkTU=23NkT. Source.
A rise in temperature increases ⟨c²⟩, leading to greater pressure at constant volume or expansion at constant pressure. Both cases signify an increase in internal energy.
Summary of Conceptual Connections
Boltzmann constant (k) relates the energy per molecule to temperature.
pV = NkT is the molecular equivalent of the macroscopic ideal gas law.
½m⟨c²⟩ = 3/2 kT expresses the average kinetic energy of gas molecules.
U = (3/2) NkT gives the total internal energy for a monatomic ideal gas.
Internal energy depends solely on temperature for an ideal gas, as intermolecular forces are assumed negligible.
These relationships form the foundation for understanding how microscopic motion manifests as macroscopic thermodynamic properties in ideal gases.
FAQ
The Boltzmann constant (1.38 × 10⁻²³ J K⁻¹) is very small because it relates microscopic energy per individual molecule to temperature.
It converts thermal energy expressed per mole (using R = 8.31 J mol⁻¹ K⁻¹) into energy per single particle, by dividing by Avogadro’s constant.
In essence:
R applies to macroscopic systems (per mole).
k applies to microscopic systems (per molecule).
Its small size simply reflects the minute amount of energy associated with one particle compared with a mole of them.
For an ideal gas, internal energy depends only on temperature because all energy is kinetic. No intermolecular forces exist, so potential energy is zero.
In real gases:
Attractive and repulsive forces cause potential energy variations.
Internal energy depends on both temperature and volume or pressure.
At low temperatures or high pressures, deviations from the ideal model become significant.
Thus, the ideal gas model simplifies energy calculations but becomes less accurate near condensation or at very high densities.
The factor 3/2 arises from the three translational degrees of freedom a molecule possesses in three-dimensional space.
Each degree of freedom (motion along x, y, and z axes) contributes an average kinetic energy of ½kT according to the equipartition of energy principle.
Adding these contributions:
Total mean kinetic energy per molecule = 3 × ½kT = 3/2 kT.
For N molecules, total internal energy = (3/2) NkT.
The Boltzmann constant links microscopic molecular behaviour to macroscopic thermodynamic quantities such as entropy.
It appears in the statistical definition of entropy:
S = k ln W
where W represents the number of possible microstates of the system.
This relation shows that k acts as a scaling factor between the microscopic multiplicity of molecular arrangements and the measurable macroscopic entropy, reinforcing its role as a bridge between scales.
Not generally. The value (3/2)NkT applies strictly to monatomic ideal gases, where only translational motion contributes to internal energy.
For diatomic or polyatomic gases, additional energy modes exist:
Rotational and vibrational motions provide extra degrees of freedom.
At higher temperatures, these modes become active, increasing the internal energy beyond (3/2)NkT.
However, at very low temperatures, rotational and vibrational modes may be “frozen out”, making their behaviour approximate that of a monatomic gas.
Practice Questions
Question 1 (2 marks)
State the relationship between the average kinetic energy of a molecule in an ideal gas and the absolute temperature. Explain how this relationship defines the Boltzmann constant.
Mark scheme:
(1 mark) Correctly states that the average kinetic energy of a molecule is proportional to its absolute temperature, using the relation ½m⟨c²⟩ = 3/2 kT.
(1 mark) Explains that the Boltzmann constant (k) is the proportionality constant linking the average kinetic energy of a molecule to its absolute temperature.
Question 2 (5 marks)
A sealed container holds an ideal monatomic gas. Using kinetic theory, derive an expression for the internal energy of the gas in terms of the number of molecules (N), the Boltzmann constant (k), and the absolute temperature (T). State any assumptions made in your derivation.
Mark scheme:
(1 mark) Starts from the microscopic expression for the pressure of an ideal gas: pV = (1/3) Nm⟨c²⟩.
(1 mark) States that the average kinetic energy per molecule is ½m⟨c²⟩ = 3/2 kT.
(1 mark) Multiplies the average kinetic energy by the total number of molecules to obtain total internal energy: U = N × ½m⟨c²⟩ = (3/2) NkT.
(1 mark) Identifies that internal energy is entirely kinetic for an ideal gas because there are no intermolecular forces and hence no potential energy.
(1 mark) States assumptions such as: gas consists of identical point molecules; collisions are elastic; and the gas behaves ideally with no interactions other than collisions.

