OCR Specification focus:
‘Explain alpha-particle scattering as evidence for a small, dense, positively charged nucleus.’
Alpha-particle scattering experiments revealed fundamental features of atomic structure. By observing how alpha particles interact with matter, physicists inferred the existence of a concentrated, positively charged nuclear core.
Rutherford Scattering and the Discovery of the Nucleus
Historical Context and Experimental Purpose
In the early twentieth century, the Rutherford–Geiger–Marsden experiment challenged the then-dominant plum-pudding model of the atom. This earlier model assumed that positive charge was spread uniformly throughout the atom. The purpose of the scattering experiment was to investigate whether this distribution of charge was correct by firing alpha particles at extremely thin metal foils (often gold) and monitoring how they deflected.
Alpha particles were known to be positively charged helium nuclei, moving at high speeds and energetic enough to pass through thin materials. Their behaviour on interacting with matter gave crucial clues about the internal structure of atoms.
Alpha particle: A helium nucleus consisting of two protons and two neutrons, carrying a +2e charge.
After this experiment was performed, it became clear that the existing atomic model could not explain observations of large-angle deflections. This realisation led directly to the nuclear model of the atom.
Experimental Setup and Observations
The scattering investigation used a source of alpha particles, a thin metallic foil, and a fluorescent screen to detect the scattered particles.
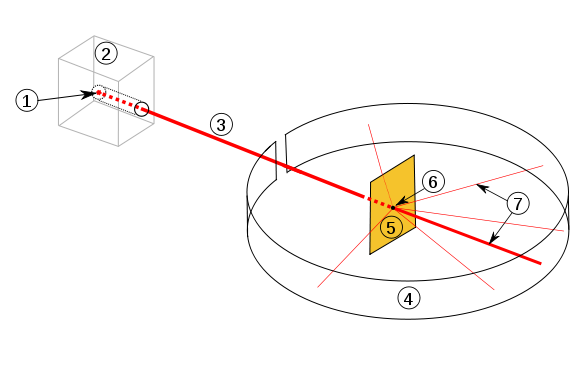
Diagram of Rutherford’s gold-foil scattering apparatus. A narrow beam of alpha particles from a radium source passes through a lead collimator, strikes a thin gold foil, and is detected on a surrounding fluorescent screen. Only a small fraction are deflected through large angles, illustrating that most of the atom is empty space and the positive charge is concentrated in a tiny nucleus. Source.
When an alpha particle struck the screen, it produced a tiny flash of light, allowing experimenters to count deflection events at various angles.
Key observations included:
Most alpha particles passed straight through the foil with little or no deflection.
Some particles were deflected by small angles, suggesting interaction with something positively charged.
A very small proportion were deflected by angles greater than 90°, with a few even rebounding almost directly backwards.
These unexpected large-angle deflections formed the core evidence for a radically new atomic structure.
Interpretation of Large-Angle Scattering
The plum-pudding model predicted only tiny deflections, because its diffuse positive charge could not exert strong repulsive forces on incoming alpha particles.
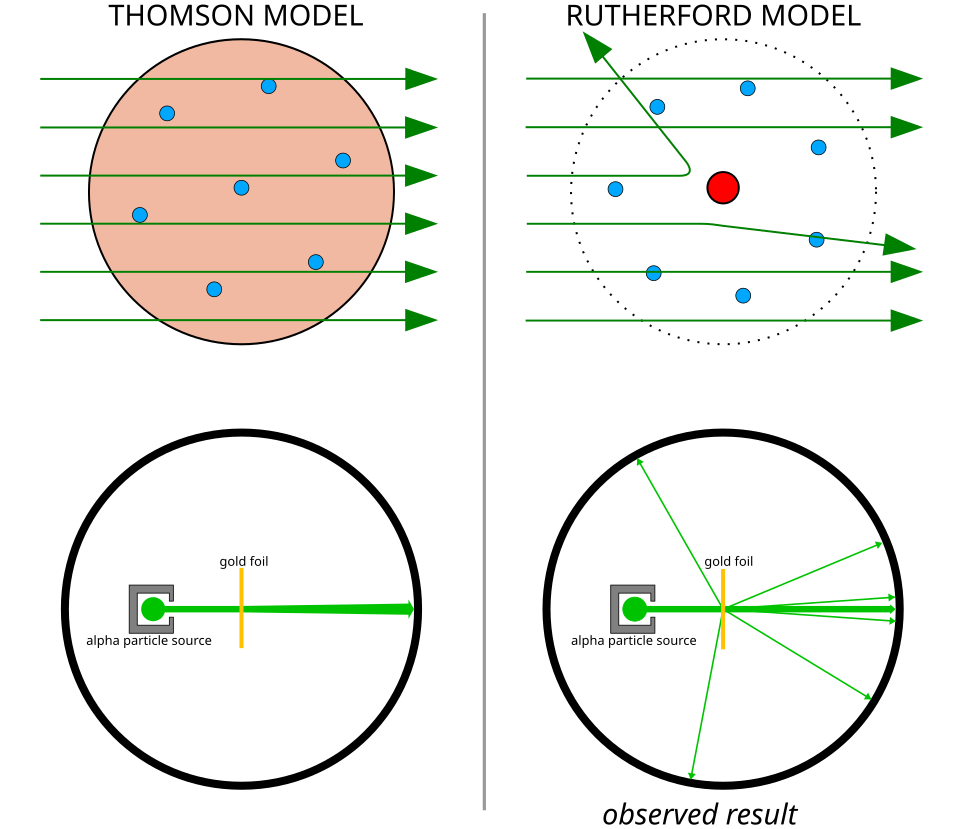
Comparison of predicted and observed alpha-particle scattering. The left panel shows the small deflections expected from the plum-pudding model, while the right panel shows the real wide-angle scattering results that required a compact, positively charged nucleus. Labels referencing Thomson’s and Rutherford’s models extend slightly beyond the OCR wording but remain helpful. Source.
Rutherford concluded that:
The atom must contain a small region of concentrated positive charge, capable of exerting strong repulsive electrostatic forces on alpha particles.
Nearly all the mass of the atom must be contained within this region, because only a massive structure could cause such dramatic deflections.
Most of the atom must be empty space, since the vast majority of particles passed through the foil unaffected.
Nucleus: The small, dense, positively charged central region of the atom containing protons and neutrons.
This nuclear model effectively explained all scattering observations and replaced the plum-pudding model.
Rutherford described the surprising backscattering events as being “as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” Such observations could not be reconciled with a diffuse distribution of charge.
The Role of Electrostatic Forces
The interaction between alpha particles and the nucleus is governed by the Coulomb force — the repulsive electrostatic force between positively charged bodies. As an alpha particle approaches a nucleus, the repulsion becomes stronger. If it approaches extremely close, the force becomes large enough to reverse its direction completely.
EQUATION
—-----------------------------------------------------------------
Coulomb’s Law (Electrostatic Force) = k Q₁Q₂ / r²
k = Coulomb constant (N m² C⁻²)
Q₁, Q₂ = interacting charges (C)
r = separation between charges (m)
—-----------------------------------------------------------------
Because the force increases rapidly as distance decreases, only an approach very near the nucleus produces significant deflection. This helps explain why only a tiny fraction of alpha particles experience large-angle scattering.
After considering the mathematical relationship between force and distance, Rutherford concluded that the nuclear radius must be extremely small compared with the radius of the atom itself. This was a critical step toward quantifying atomic structure.
Evidence for a Small, Dense, Positively Charged Nucleus
The scattering results provide conclusive evidence for three key features:
Small size: The rarity of extreme deflections implies that the nucleus occupies only a minuscule fraction of atomic volume.
Positive charge: Alpha particles were repelled, not attracted, revealing that the nucleus carries a positive charge.
High density: Significant deflections require a massive interacting body, concentrated into a very small region.
These deductions underpin the modern nuclear atom, in which electrons orbit a compact nucleus composed of protons and neutrons. Rutherford’s model marked the foundation of nuclear physics and remains essential to understanding atomic interactions.
Layered Structure of Evidence from the Scattering Experiment
Direct evidence:
Backscattering and large-angle deflections
Rare but significant events indicating a highly localised charge concentration
Indirect evidence:
Majority of alpha particles travelling undeflected
Indicates that the atom is largely empty space
Logical inference:
Positive nucleus must exist to repel positively charged alpha particles
Greater deflections correspond to closer approaches and stronger forces
This layered reasoning forms a central part of how students should understand the experimental basis of the nuclear model.
Why the Nuclear Model Replaced Earlier Models
The success of the Rutherford model lay in its ability to explain all scattering outcomes using well-established physical principles. Unlike earlier models, it accounted for both tiny and enormous deflections within a single framework. Because of this, the nuclear atom rapidly became the accepted description of atomic structure, setting the stage for later developments in quantum theory and particle physics.
FAQ
Gold can be hammered into extremely thin sheets, often just a few hundred atoms thick, without tearing. This allows alpha particles to pass through with minimal absorption.
A very thin foil ensures that scattering events are mostly due to single interactions with individual nuclei, avoiding multiple deflections that would complicate interpretation.
Gold atoms also have large nuclear charge, increasing the likelihood of observable deflections and making the experiment more sensitive to nuclear structure.
Detection relied on tiny flashes of light known as scintillations. Each alpha particle striking the zinc sulphide coating produced a faint but visible spark.
Observers used a low-power microscope to count these flashes manually. The process required working in darkened conditions and demanded considerable patience and precision.
Each flash corresponded to one particle
Counts at different angles created a scattering distribution pattern
Existing models assumed atoms were diffuse spheres of positive charge, meaning any deflection should be small. A backward rebound requires a massive, highly localised source of repulsion.
The only plausible explanation was that nearly all the atom’s mass and positive charge were concentrated in a volume far smaller than predicted. This contradicted decades of accepted theory.
The discovery forced a radical shift from a smeared-out atomic structure to one dominated by a compact nucleus.
Higher-energy alpha particles travel faster and spend less time in the strong electric field near the nucleus, reducing the angle by which they are deflected.
Lower-energy particles experience stronger turning effects because the repulsive force has more time to act.
Lower energy → larger average deflection angles
Higher energy → more forward scattering
Extreme backscattering requires a combination of close approach and sufficiently slow speed
This energy dependence helped confirm that the force responsible was electrostatic.
Although it explained scattering results, it could not account for atomic stability. Classical physics predicted orbiting electrons would radiate energy and spiral into the nucleus.
The Rutherford model also could not explain discrete spectral lines or quantised electron energies.
These limitations led to the Bohr model and later quantum mechanical descriptions, but Rutherford’s nuclear insight remained foundational.
Practice Questions
Question 1 (2 marks)
State two observations from the Rutherford alpha-particle scattering experiment and explain how each observation provides evidence for the nuclear model of the atom.
Question 1 (2 marks)
Award 1 mark for any valid observation and 1 mark for its correct explanation.
Most alpha particles passed straight through the foil (1) because most of the atom is empty space (1).
A small number of alpha particles were deflected by small angles (1) because they passed close to a positively charged nucleus and experienced electrostatic repulsion (1).
A very tiny proportion were deflected by angles greater than 90 degrees (1) because the nucleus contains most of the atom’s mass in a very small, dense, positively charged region (1).
(Max 2 marks)
Question 2 (5 marks)
Alpha particles were fired at a thin gold foil in the Geiger–Marsden experiment.
Explain, using ideas about charge and atomic structure, why:
(a) most alpha particles passed straight through the foil,
(b) some alpha particles were deflected by small angles, and
(c) a very small number were deflected through angles greater than 90 degrees.
Your answer should refer to forces acting on the alpha particles and the distribution of mass and charge inside the atom.
Question 2 (5 marks)
Award marks for each correct explanation point.
(a) Most pass straight through:
Atoms consist mostly of empty space (1).
Alpha particles do not encounter anything dense enough to deflect them (1).
(b) Small-angle deflections:
Alpha particles are positively charged (1).
They experience a repulsive electrostatic force when they pass near the positively charged nucleus (1).
(c) Large-angle deflections:
Only strong repulsive forces at very short distances can produce deflections greater than 90 degrees (1).
This is evidence that the nucleus is very small, dense, and contains most of the atom’s mass (1).
The large-angle scattering confirms the concentration of positive charge in the nucleus (1).
(Max 5 marks)

