AP Syllabus focus:
‘Comparison of various experimental designs and methods, such as completely randomized designs, single-blind and double-blind experiments, control groups, and the placebo effect. The section will cover the rationale behind each design/method and its implications for experimental integrity.’
Design choices in experiments strongly influence the reliability of conclusions. Understanding common experimental designs and methods helps ensure valid comparisons, reduce bias, and strengthen causal claims.
Experimental Designs and Methods
Completely Randomized Designs
A completely randomized design assigns all experimental units to treatments using a random process. This structure ensures that each unit has an equal chance of receiving any treatment, supporting strong causal inference by distributing variability from uncontrolled factors across groups.
Completely Randomized Design: An experimental design in which all experimental units are randomly assigned to available treatment groups without consideration of any characteristics of the units.
In this approach, randomization protects against systematic differences between groups, meaning that any observed difference in the response variable can reasonably be attributed to the treatment.
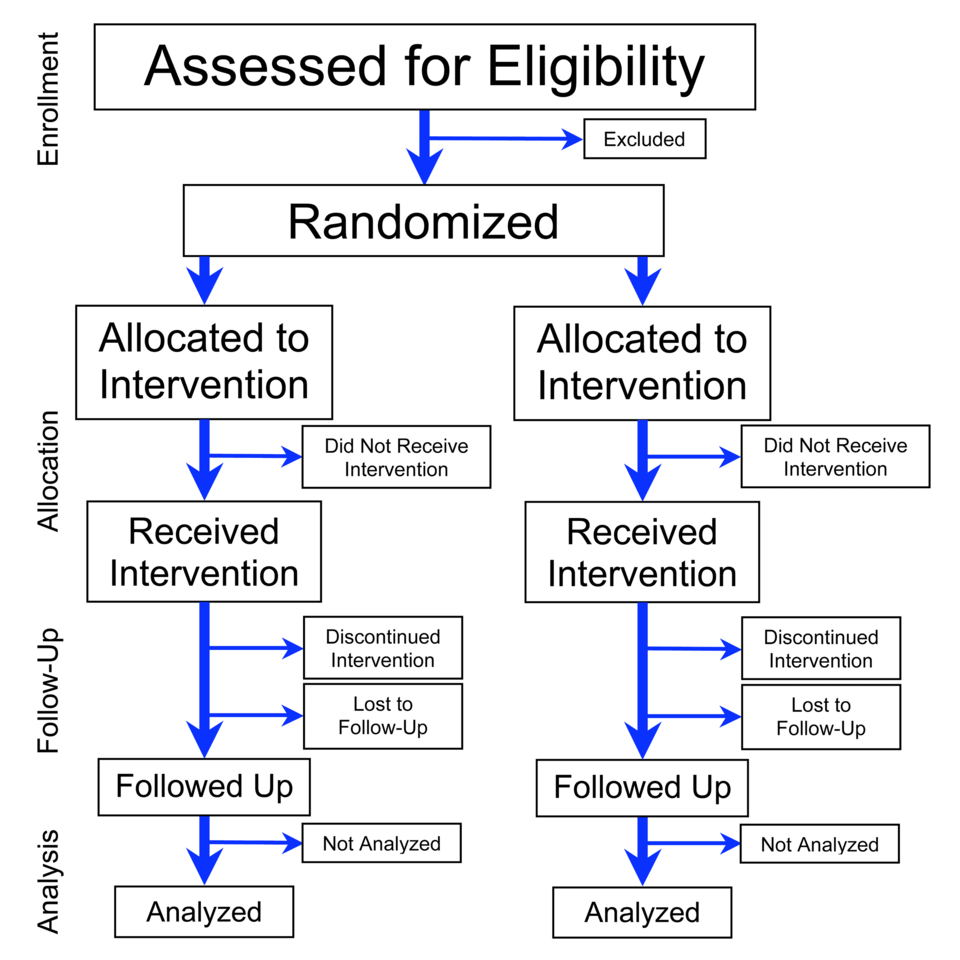
A flowchart illustrating the phases of a parallel randomized trial from enrollment through allocation, follow-up, and analysis. The diagram emphasizes random assignment and the separation of treatment groups. Extra details such as participant loss over time extend beyond the AP syllabus but help visualize how real experiments track subjects. Source.
This design is most appropriate when experimental units are relatively similar and no obvious subgrouping structure exists.
Control Groups and Treatment Structure
Experiments frequently include a control group, which serves as a baseline for comparison. The control group either receives no treatment or receives a standard condition against which the effectiveness of new treatments can be measured.
Control Group: A group in an experiment that receives no treatment or a standard treatment to provide a basis for comparison with experimental groups.
Including a control group helps isolate the effect of the explanatory variable by ensuring that the only systematic difference between groups is the treatment received.
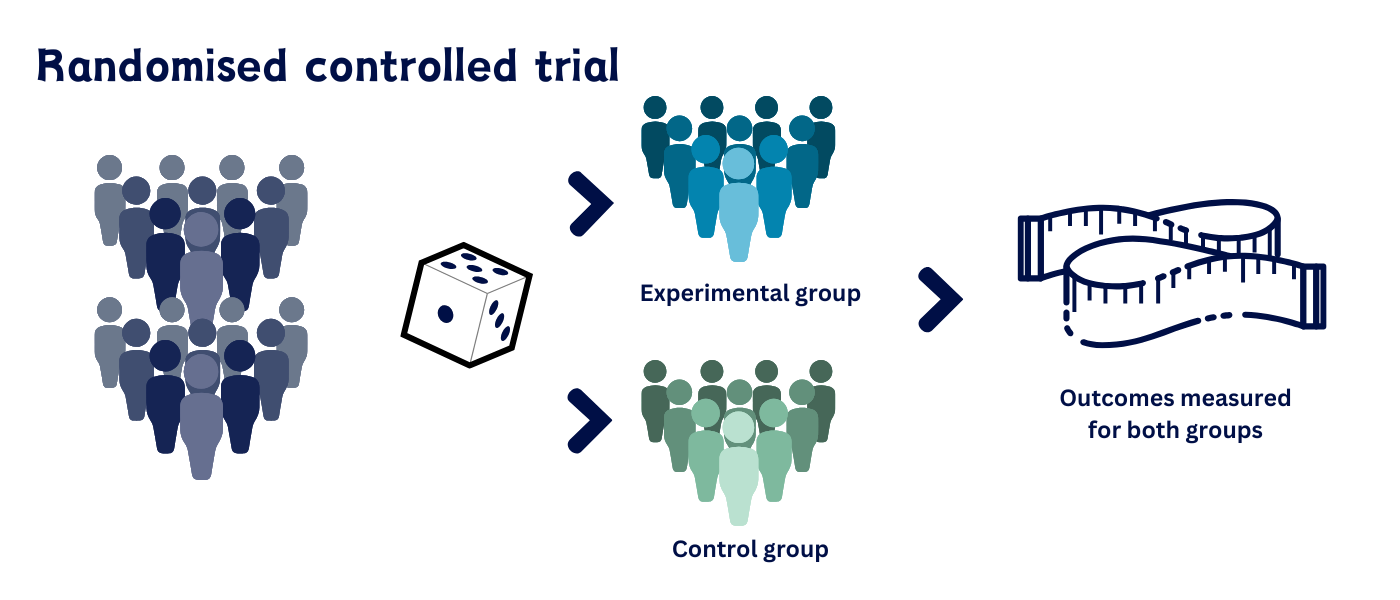
A conceptual diagram showing participants being randomly divided into an experimental group and a control group. The die emphasizes that chance determines treatment assignment. The image focuses on group formation and does not include later steps of the experiment. Source.
Good experimental practice uses control groups to separate true treatment effects from natural variability or external influences.
Placebo and the Placebo Effect
In experiments involving human subjects, researchers often use a placebo, a treatment with no active ingredient, to measure psychological or expectation-driven effects.
Placebo Effect: A change in a participant’s response caused solely by the expectation of receiving a treatment rather than by the treatment itself.
A placebo allows researchers to determine whether improvements occur because of the treatment or because participants believe they are receiving something beneficial. Identifying this distinction is essential for evaluating true treatment impact in medical, behavioral, and consumer research.
Blinding in Experiments
Blinding is a method used to limit bias by preventing participants, researchers, or both from knowing which treatment is being administered.
Single-blind experiment:
Only the participants (or sometimes the researchers measuring outcomes) do not know which treatment they receive.Double-blind experiment:
Both participants and the individuals administering or evaluating responses are unaware of treatment assignments.
Double-Blind Experiment: An experiment in which neither participants nor the researchers who interact with them or measure outcomes know which treatment each subject receives.
This structure reduces response bias and experimenter bias, improving the credibility of observed treatment effects. Double-blind designs are especially valuable when measuring subjective outcomes or when expectations could influence results.
Rationale Behind These Methods
Experimental designs and methods are chosen intentionally to enhance validity, limit confounding, and support strong causal conclusions. Several key rationales underlie these practices:
Randomization distributes uncontrolled variability and reduces the likelihood of systematic differences between treatment groups.
Control groups provide a meaningful baseline to assess whether treatments produce measurable change.
Placebos help distinguish physiological effects from psychological expectations.
Blinding prevents participants and researchers from influencing outcomes, intentionally or unintentionally.
Together, these choices strengthen internal validity—the degree to which the experiment demonstrates a causal relationship.
Implications for Experimental Integrity
Using proper experimental designs increases the reliability of conclusions and the defensibility of causal claims. High-quality studies integrate multiple methods:
A completely randomized design ensures fairness and comparability between treatment groups.
A control group anchors interpretation of observed results.
A placebo and appropriate blinding eliminate alternative explanations related to expectations or measurement bias.
These practices guard against misleading conclusions by ensuring that observed differences are due to the treatment and not external influences, personal beliefs, or researcher expectations.
When to Use Each Method
Different research contexts require different combinations of experimental strategies:
Use a completely randomized design when units are similar, and there is no need to account for subgroups.
Include a control group whenever a baseline comparison is required.
Use a placebo in experiments involving subjective or expectation-driven responses.
Apply single-blind or double-blind techniques when awareness of treatment could influence behavior or measurement.
Selecting the appropriate design elements ensures that the experiment aligns with its objectives and supports valid inference about treatment effects.
FAQ
A completely randomised design is most useful when experimental units are sufficiently similar that subgrouping or blocking offers little advantage. This allows all variability that is not due to the treatment to be spread evenly across groups through random assignment.
It is also preferred when the researcher seeks simplicity, as it requires fewer structural decisions than blocked or matched designs.
A placebo is generally used when participants’ expectations could alter their responses, particularly in studies involving pain, emotions, or subjective health measures.
A placebo is less necessary in settings where expectations cannot meaningfully influence outcomes, such as chemical reactions or automated mechanical processes.
An effective control group should be treated identically to the treatment group in every respect except the treatment itself.
Key features include:
• Identical measurement procedures
• Similar environmental or testing conditions
• Clear definition of what constitutes ‘no treatment’ or a standard condition
Blinding may be impractical when the treatment is inherently obvious, such as comparing two visibly different teaching methods or physical training programmes.
It can also be inappropriate when safety requires participants to know the treatment they receive, for example with strong medications or hazardous materials.
While random assignment can produce unequal group sizes, researchers can reduce the chance of imbalance by using restricted randomisation methods, such as fixed block randomisation.
Alternatively, they may simply increase the total sample size, which reduces the impact of any imbalance on the validity of the results.
Practice Questions
Question 1 (1–3 marks)
A researcher conducts a completely randomised experiment to test whether a new fertiliser increases plant growth. Thirty identical plants are randomly assigned to either the treatment group (new fertiliser) or the control group (no fertiliser).
a) Identify the purpose of random assignment in this experiment. (1 mark)
b) Explain why including a control group is important in this context. (1–2 marks)
Question 1
a) 1 mark
• States that random assignment helps ensure groups are similar or comparable before treatment OR reduces bias OR distributes uncontrolled variables evenly across groups.
(Any one earns the mark.)
b) Up to 2 marks
• 1 mark for stating that the control group provides a baseline for comparison.
• 1 mark for explaining that it allows the researcher to isolate the effect of the fertiliser or determine whether changes in plant growth are due to the treatment rather than natural variation or external factors.
Question 2 (4–6 marks)
A medical team is evaluating a new headache medication. They design a double-blind experiment in which participants are randomly assigned to receive either the new medication or a placebo. Neither the participants nor the staff administering the pills know which treatment is being given.
a) Explain why a placebo is used in this study. (1–2 marks)
b) Describe how the double-blind design strengthens the validity of the results. (2–3 marks)
c) State one limitation of this experimental design. (1 mark)
Question 2
a) Up to 2 marks
• 1 mark for stating that the placebo controls for psychological or expectation effects.
• 1 mark for explaining that it helps determine whether improvements are caused by the medication itself rather than participants’ beliefs.
b) Up to 3 marks
• 1 mark for stating that neither participants nor administrators know who receives which treatment.
• 1 mark for explaining that this prevents participants’ responses from being influenced by knowing their treatment.
• 1 mark for explaining that it prevents researcher bias in administering treatment or measuring outcomes.
c) 1 mark
• Identifies a reasonable limitation, such as ethical concerns, cost, participant drop-out, difficulty generalising results, or that double-blind procedures may be impractical in some settings.

