OCR Specification focus:
‘Use appropriate SI units for all measurements, showing clear unit conversions where needed, and recording values consistently.’
Introduction
Choosing and using appropriate units is fundamental to reliable experimental work in chemistry. Accurate measurement, clear recording, and correct conversions ensure data integrity and meaningful comparison between results.
The Role of Units in Scientific Measurement
Units provide a standardised system for expressing quantities, allowing scientists to communicate and compare results universally. In chemistry, measurements such as mass, volume, temperature, pressure, and concentration must be recorded using recognised units, primarily from the Système International d’Unités (SI units).
Without standardised units, experimental data lose reliability and cannot be compared or analysed correctly. This subsubtopic focuses on how to select, convert, and use units consistently when collecting and processing data.
The SI System
The SI system (Système International d’Unités) is the globally accepted metric system that ensures consistency and precision in scientific measurement. It is based on seven base units, from which all other derived units are formed.
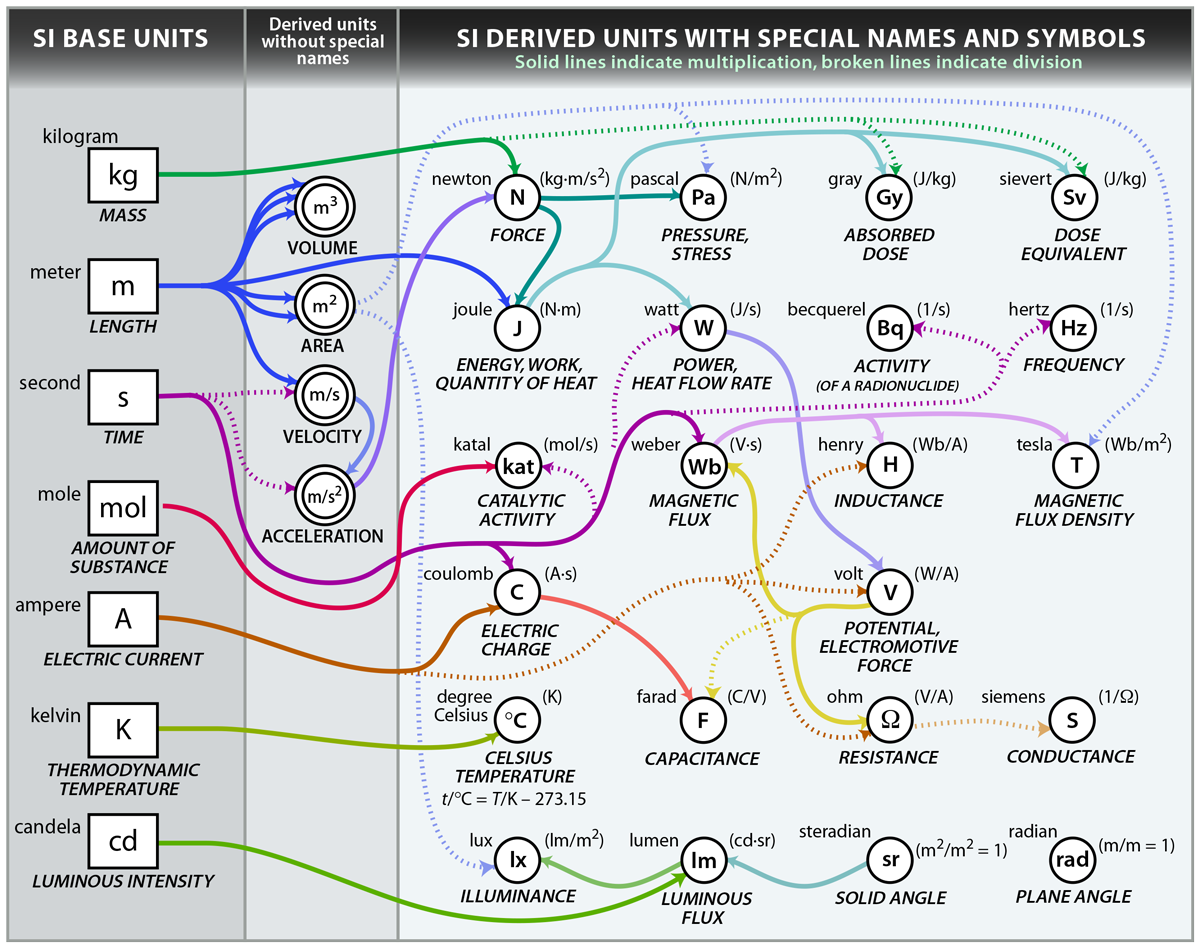
Diagram illustrating the seven SI base units (in rectangles) and key derived units (in circles) with arrows showing their derivation. Extra detail: includes many derived units beyond those commonly used in A‑Level chemistry (e.g., katal, sievert). Source
Base SI Units
Mass: kilogram (kg)
Length: metre (m)
Time: second (s)
Temperature: kelvin (K)
Amount of substance: mole (mol)
Electric current: ampere (A)
Luminous intensity: candela (cd)
All other units used in chemistry, such as joules (J) for energy or newtons (N) for force, are derived from these base units.
Derived Units in Chemistry
Many chemical measurements involve derived units, which are combinations of the base SI units. These include:
Energy: joule (J) — derived from kg·m²·s⁻²
Pressure: pascal (Pa) — equal to N·m⁻² or kg·m⁻¹·s⁻²
Concentration: mole per cubic decimetre (mol dm⁻³)
Volume: cubic metre (m³) or cubic decimetre (dm³)
Density: kilogram per cubic metre (kg m⁻³)
Density (ρ) = Mass (m) ÷ Volume (V)
ρ = Density (kg m⁻³)
m = Mass (kg)
V = Volume (m³)
When recording or converting units, it is essential that derived units remain dimensionally consistent throughout all calculations and data processing.
Appropriate Unit Selection
Ensuring Measurement Suitability
Selecting the correct unit depends on:
The magnitude of the quantity being measured
The precision required by the experimental procedure
The apparatus used for measurement
For example:
A burette measures volume accurately in cm³ or mL, while dm³ is used when calculating molar concentrations.
A balance typically records mass in grams (g), which may then be converted to kilograms (kg) for calculations involving energy or density.
Using a consistent system avoids confusion and improves data comparability across different experimental stages.
Unit Conversions
Converting Between Common Units
Chemistry often requires conversion between related units, especially when combining data from different instruments or sources.
Typical conversions include:
1 dm³ = 1000 cm³ = 1 L
1 m³ = 1000 dm³ = 10⁶ cm³
1 kJ = 1000 J
1 °C = 273.15 K (for temperature conversion)
When converting units, it is crucial to maintain significant figures and ensure that prefixes (e.g., milli-, centi-, kilo-) are used appropriately.
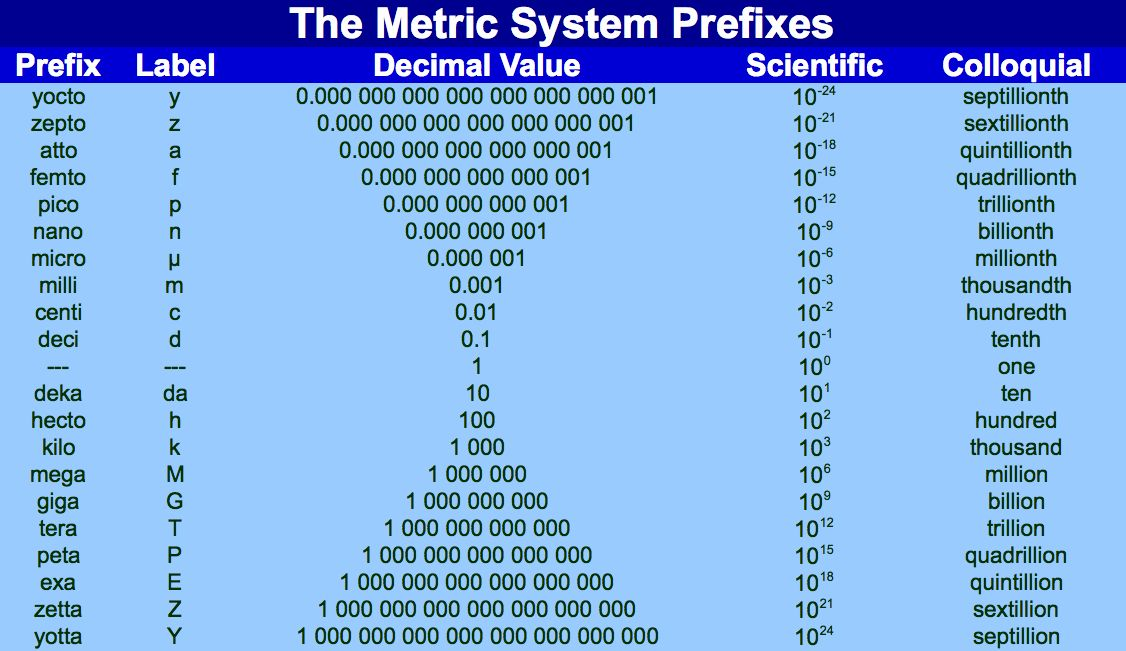
Table of metric (SI) prefixes showing the prefix, symbol and power of ten factor. Extra content: includes prefixes beyond those typically required for A‑Level (such as femto‑, atto‑), which can be ignored for now. Source
Significant Figures: The digits in a number that represent meaningful measurement accuracy, including all certain digits plus the first uncertain digit.
Errors in unit conversion can lead to major discrepancies in final results, particularly when working with concentrations, gas volumes, or thermodynamic data.
Recording Units Consistently
Data Presentation
When recording experimental results:
Always include units in data tables and column headings.
Use consistent units throughout — never mix, for example, cm³ and dm³ in the same dataset.
Clearly label axes on graphs with the quantity and unit, e.g., Temperature / K or Concentration / mol dm⁻³.
Use standard abbreviations recognised by the SI system.
Consistency prevents misinterpretation and ensures calculations are traceable and verifiable.
Non-SI Units in Chemistry
Although SI units are preferred, some non-SI units remain in common chemical use for historical or practical reasons. These include:
Litres (L) for volume (1 L = 1 dm³)
Atmospheres (atm) for pressure (1 atm = 101,325 Pa)
Degrees Celsius (°C) for temperature, although kelvin (K) is required for calculations
When using non-SI units, always convert to SI before performing any calculations to ensure accuracy and standardisation.
Dimensional Analysis
Dimensional analysis is a method of checking that units in an equation are consistent on both sides. This ensures that the equation is physically valid and calculations are reliable.
Dimensional Analysis: The process of verifying the correctness of equations or calculations by ensuring that units are consistent and equivalent throughout.
For example, in the ideal gas equation pV = nRT, the units on both sides must reduce to J (kg·m²·s⁻²) when expressed in base SI terms.
Recording and Reporting Uncertainty in Units
Every measurement includes uncertainty, which should be expressed in the same unit as the measurement itself. For instance:
A mass reading might be 12.53 ± 0.01 g
A volume reading might be 25.00 ± 0.05 cm³
Always record uncertainties alongside values to communicate precision clearly. The uncertainty helps in evaluating reliability and determining percentage error.
Best Practice in Unit Application
To ensure accuracy and consistency when using units:
Always use SI units unless otherwise stated.
Record all data with units at every stage.
Convert units before analysis, not after.
Check dimensional consistency in every equation used.
Maintain appropriate significant figures according to instrument precision.
By adhering to these principles, students can ensure that their experimental data meet the expectations of the OCR specification — precise, consistent, and scientifically valid.
FAQ
SI units provide a universal standard that eliminates ambiguity between measurements taken in different laboratories or countries. This consistency is essential when comparing data, performing calculations, or interpreting results.
Using SI units also helps ensure that equations remain dimensionally consistent and that derived quantities, such as energy or pressure, are expressed correctly. Converting to SI before analysis minimises systematic errors and supports scientific reproducibility.
Prefixes allow values to be written in a convenient numerical range without excessive zeros, making them easier to read and compare.
For example:
0.001 m becomes 1 mm
1000 g becomes 1 kg
Using appropriate prefixes reduces rounding errors and helps align data with the precision of the measuring instrument, ensuring accuracy and clarity in reports.
Students often:
Forget to square or cube conversion factors when dealing with area or volume.
Mix non-SI and SI units within the same calculation.
Round too early, losing precision.
To avoid these, always write conversion factors explicitly, check dimensional consistency, and delay rounding until the final step of a calculation.
Reporting too many significant figures suggests a false level of accuracy, while too few obscure real precision.
The number of significant figures should reflect the smallest division or uncertainty on the measuring device. For instance, if a balance reads to 0.01 g, the recorded mass should also be reported to two decimal places. This ensures that recorded data accurately represents the instrument’s reliability.
Dimensional analysis checks that units on both sides of an equation are consistent. If the units do not match, a calculation or substitution error has occurred.
For example, if energy (J) is calculated and the resulting unit is kg·m·s⁻¹, a missing time factor is indicated.
By verifying that each term carries the correct units before solving, dimensional analysis prevents conceptual mistakes and confirms the physical validity of the equation used.
Practice Questions
When measuring the volume of a liquid using a burette, explain why it is important to record the result in cm³ or mL rather than in L. 2 marks
1 mark: Recognises that cm³ or mL provide greater precision due to smaller unit size.
1 mark: States that using L would reduce accuracy or make small changes harder to detect.
A student measures the temperature change in a reaction using a thermometer that records to the nearest 0.1 °C. The initial temperature is 20.4 °C and the final temperature is 25.6 °C.
(a) Calculate the temperature change and express it in both °C and K. (2 marks)
(b) Explain why it is appropriate to record both temperatures to one decimal place. (2 marks)
(c) State why the kelvin (K) is preferred over degrees Celsius (°C) for calculations in thermodynamics. (1 mark)
5 marks
(a)
1 mark: Correct temperature change = 5.2 °C.
1 mark: Correct conversion = 5.2 K.
(b)
1 mark: Recognises that readings should be recorded to the same decimal place as the instrument’s precision.
1 mark: States that this ensures consistency and avoids implying greater accuracy than the apparatus allows.
(c)
1 mark: Explains that the kelvin is an absolute temperature scale starting at absolute zero, making it suitable for thermodynamic calculations.

