OCR Specification focus:
‘Use a wide range of practical apparatus and techniques correctly, as outlined in the specification and Practical Endorsement requirements.’
Introduction
Competent use of practical apparatus and techniques is central to OCR A-Level Chemistry. Mastering accurate, safe, and efficient laboratory practices ensures reliable data collection and strong practical endorsement performance.
Understanding the Role of Practical Techniques
Correct use of apparatus and techniques underpins experimental accuracy, precision, and reproducibility. Students must demonstrate competency across a broad range of laboratory skills specified in the OCR Practical Endorsement.
Core Practical Equipment
Students are expected to handle apparatus confidently and safely. This includes:
Measuring instruments – burettes, pipettes, measuring cylinders, and volumetric flasks.
Heating equipment – Bunsen burners, hot plates, and water baths.
Cooling and condensation apparatus – Liebig condensers, ice baths, and reflux setups.
Filtration and separation tools – filter funnels, Buchner funnels, and centrifuges.
Balances and weighing boats for precise mass measurements.
Each item requires correct calibration, reading, and maintenance to ensure measurement validity and prevent systematic error.
Using Glassware Accurately
When preparing or measuring solutions:
Always read the meniscus at eye level on the calibration mark.
Use pipette fillers for safety rather than mouth pipetting.
Rinse apparatus with the solution to be used to avoid contamination.
Meniscus: The curved surface of a liquid in a container, caused by surface tension, which should be read at its lowest point for aqueous solutions.
Consistency in technique prevents random errors and improves precision.
Heating and Cooling Techniques
Heating substances evenly avoids thermal gradients that may distort results. Safe heating practices include:
Ensuring flammable substances are kept away from naked flames.
Using a water bath for heating volatile liquids.
Employing anti-bumping granules during boiling to prevent splashing.
When cooling reactions:
Use ice baths for temperature control.
Avoid thermal shock by gradually lowering temperature for glassware.
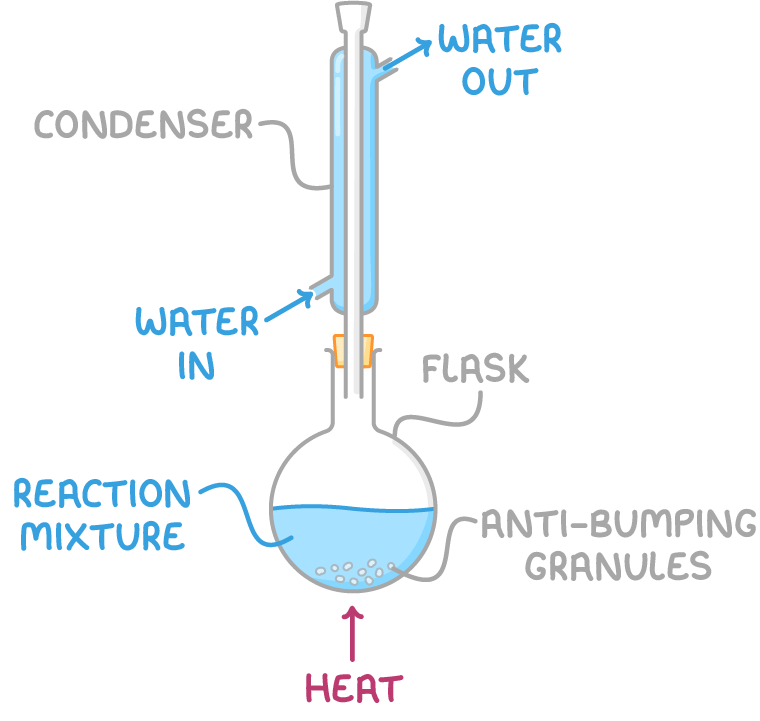
Labelled diagram of a reflux setup showing a round-bottom flask with anti-bumping granules, and a vertical Liebig condenser with water in (bottom) and water out (top). It supports understanding of safe, correct heating practices. (Some detail shown extends into organic-chemistry technique rather than purely general OCR practical skills.) Source
Thermal Gradient: The rate of temperature change over a distance within a substance or system.
Controlled heating and cooling maintain reaction consistency and protect apparatus integrity.
Techniques for Quantitative Experiments
Correct use of apparatus is vital for titration and gravimetric analysis.
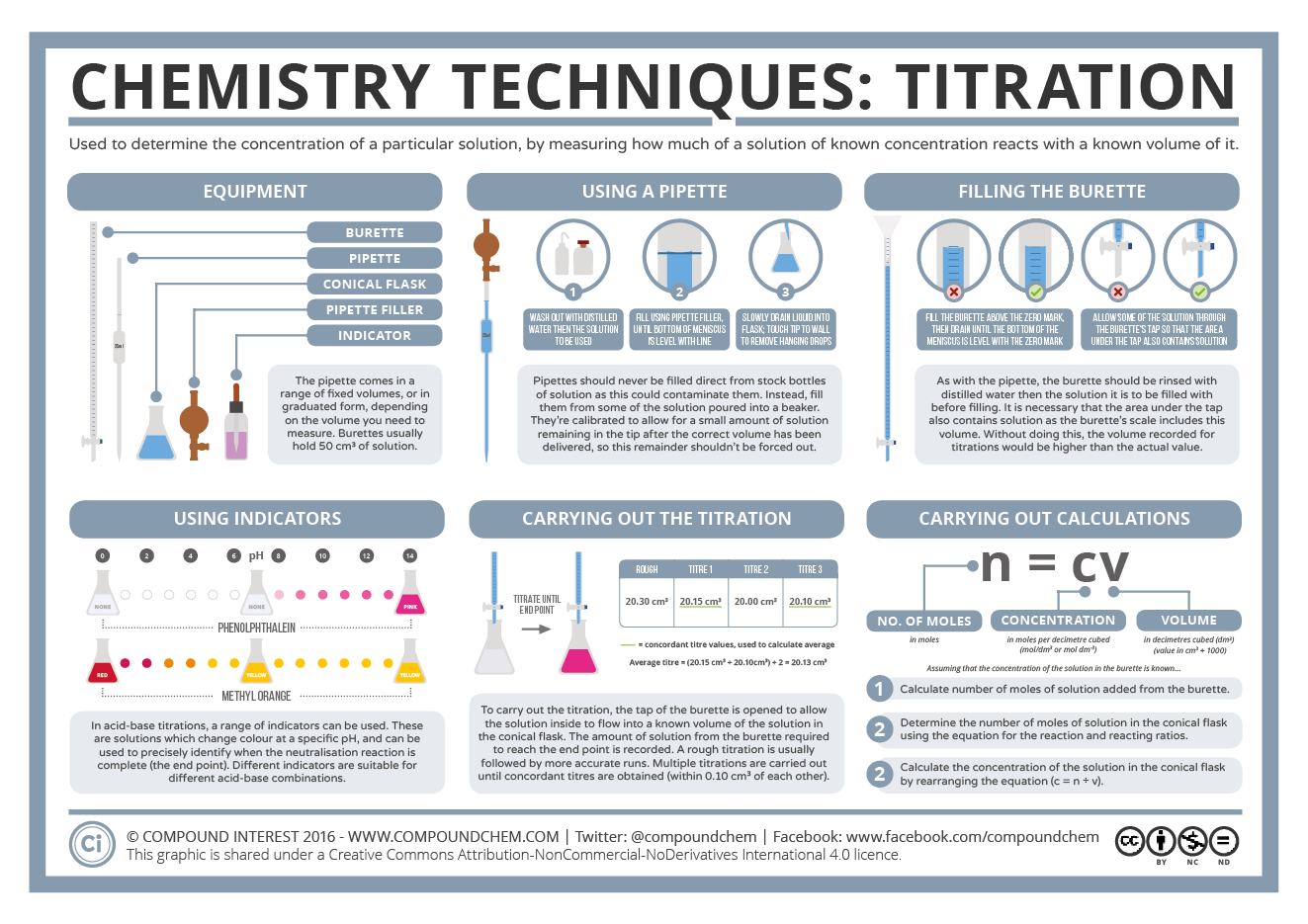
Diagram of a titration setup (burette, pipette, conical flask and clamp stand). It illustrates correct arrangement and labelling of volumetric apparatus for accurate measurement. (Note: this particular diagram is designed for a generic titration and may include additional labelling beyond the OCR-specific requirements.) Source
For titration:
Rinse and fill burettes and pipettes appropriately.
Remove air bubbles before starting.
Read volumes to ±0.05 cm³ accuracy at eye level.
For gravimetric work:
Use analytical balances with a precision of ±0.001 g.
Avoid draughts when weighing.
Cool crucibles before reweighing to prevent convection errors.
Gravimetric Analysis: A method of quantitative chemical analysis involving measurement of mass.
Maintaining accuracy in measurement ensures valid and reproducible outcomes.
Techniques for Qualitative Experiments
Qualitative techniques often involve observation and identification of chemical changes. Students must:
Record colour changes, gas evolution, and precipitate formation accurately.
Use spotting tiles for small-scale reactions.
Employ fume cupboards when handling volatile or hazardous reagents.
Clear and systematic observation supports accurate interpretation of results and safe laboratory conduct.
Filtration, Distillation and Reflux
Correct setup and operation of apparatus for separation and purification are essential.
Filtration removes solids from liquids using filter paper or vacuum suction.
Distillation separates liquids by boiling point differences; thermometers must be positioned correctly at the neck of the flask.
Reflux allows heating of volatile mixtures without loss of reactants, using vertical condensers and water flow from bottom to top.
Reflux: The continuous boiling and condensation of a reaction mixture to ensure completion without loss of volatile components.
Attention to apparatus setup directly affects reaction yield and purity of products.
Measurement and Calibration
Accuracy relies on correctly calibrated instruments and awareness of potential deviations.
Verify calibration marks on volumetric glassware.
Check thermometers and pH meters against known standards.
Use zero error corrections on balances and measuring devices.
Zero Error: The systematic error that occurs when an instrument does not read zero when no quantity is being measured.
Calibrated instruments minimise systematic error and improve data reliability.
Safe and Effective Technique Application
Chemistry practicals demand adherence to Health and Safety Executive (HSE) standards.
Key principles include:
Wearing goggles and lab coats at all times.
Handling acids and alkalis using appropriate PPE.
Disposing of waste according to chemical hazard classification.
Using tongs or heatproof mats with hot apparatus.
Proper safety ensures valid experimental performance and compliance with the Practical Endorsement criteria.
Recording Practical Competence
In the OCR Practical Endorsement, students must demonstrate proficiency across the twelve common practical techniques, which include:
Measuring and mixing liquids accurately.
Using qualitative tests for ions and organic compounds.
Conducting quantitative analyses such as titrations.
Employing heating, cooling, and distillation techniques.
Teachers assess and sign off competence when students perform techniques safely, systematically, and independently.
Linking Techniques to Experimental Success
Proficiency in apparatus use supports reliable data collection and meaningful analysis. Inaccurate readings, improper setup, or misuse of apparatus cause systematic error, compromise accuracy, and reduce the validity of conclusions.
Validity: The extent to which experimental results and conclusions accurately reflect the true situation being studied.
Ultimately, correct use of practical apparatus and techniques forms the foundation for all experimental chemistry, bridging theoretical knowledge with precise, evidence-based practice.
FAQ
Systematic errors often result from miscalibrated instruments or consistent procedural faults. For example, a burette that doesn’t read zero correctly introduces a fixed offset in all readings. Similarly, using contaminated glassware can bias concentration values.
To minimise systematic error:
Always check for zero errors on balances and burettes.
Calibrate pH meters, thermometers, and volumetric instruments regularly.
Rinse apparatus with the solution it will contain to avoid dilution.
Rinsing ensures that no residue or water remains that could alter the concentration of solutions used. For example, rinsing a pipette with distilled water would dilute the solution being transferred, while rinsing it with the solution ensures concentration consistency.
Always rinse:
Burettes and pipettes with the solution they’ll hold.
Conical flasks with distilled water only, to avoid altering reactant concentrations.
Before heating, inspect equipment for cracks or leaks that may cause accidents. Ensure gas tubing and Bunsen burners are intact and correctly connected.
Key checks:
Verify that the area is free from flammable substances.
Use heatproof mats and clamps to secure glassware.
Check for appropriate ventilation or use a fume cupboard when heating volatile liquids.
Anti-bumping granules ensure smooth boiling by providing nucleation sites for vapour bubbles. Without them, liquid may superheat and release vapour suddenly, leading to dangerous splashing or bumping.
This promotes:
Safer heating, reducing the risk of burns or broken glassware.
More consistent temperature distribution, improving reaction reliability.
Repeatability depends on maintaining consistent technique across measurements. Use the same person and method for reading menisci and filling apparatus to reduce variation.
Additional steps include:
Reading at eye level to prevent parallax error.
Using clean, dry apparatus each time.
Recording readings to the same decimal place, reflecting the precision of the measuring instrument.
Practice Questions
A student is preparing to carry out a titration to determine the concentration of an acid solution. Describe two safety precautions the student should take when using a burette and pipette. (2 marks)
(1 mark) Uses a pipette filler instead of mouth suction.
(1 mark) Ensures eye-level reading of meniscus to avoid parallax error and spillage.
A student is asked to heat a reaction mixture under reflux to ensure the reaction goes to completion.
(a) Describe how the reflux apparatus should be set up and used correctly. (3 marks)
(b) Explain why refluxing is preferred over simple boiling for this type of reaction. (2 marks)
(5 marks)
(a)
(1 mark) Round-bottom flask used with anti-bumping granules.
(1 mark) Condenser fitted vertically, with water entering at the bottom and leaving at the top.
(1 mark) Apparatus assembled so vapour condenses and returns to the flask without loss of reactants.
(b)
(1 mark) Prevents loss of volatile reactants or products by condensation.
(1 mark) Allows the mixture to be heated continuously until completion of the reaction without evaporation losses.

