OCR Specification focus:
‘Current is due to electrons moving in metals and ions moving in electrolytes.’
Electric current arises from the movement of charged particles called charge carriers. In metals, these carriers are electrons, while in electrolytes, ions transport charge through the solution.
Charge Carriers and the Nature of Electric Current
Electric current is the rate of flow of electric charge through a conductor. The movement of charge carriers enables energy transfer and allows electric circuits to function effectively. Understanding the type of charge carriers in different materials—specifically metals and electrolytes—is crucial to explaining how current flows and behaves in various systems.
Electric Current: The rate at which electric charge flows through a conductor, measured in amperes (A).
In all conductors, the flow of charge is a macroscopic effect of the microscopic motion of charged particles. The ease with which these particles move depends on the material’s structure and the nature of its bonding.
Charge Carriers in Metals
In metals, electric current is carried almost entirely by electrons. The atoms in metallic solids are arranged in a giant lattice structure, where each metal atom contributes one or more delocalised electrons that are free to move throughout the lattice. These delocalised electrons form what is sometimes described as a ‘sea of electrons’ surrounding the fixed, positively charged metal ions.
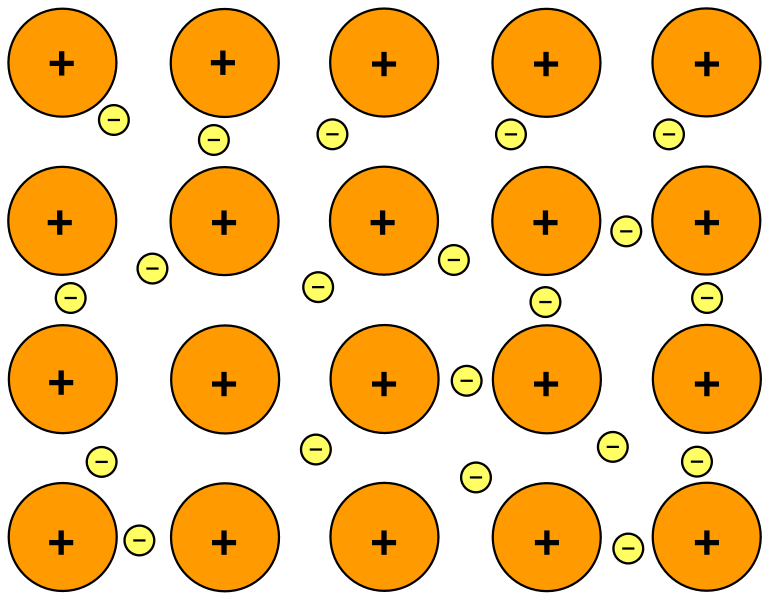
Schematic of metallic bonding, showing a regular array of positive metal ions immersed in a sea of delocalised electrons. The ions are fixed while electrons are mobile, carrying charge through the metal. This simplified model emphasises charge carriers rather than atomic detail. Source.
Delocalised Electron: An electron in a metallic structure that is not bound to any specific atom and can move freely through the lattice.
When a potential difference is applied across a metallic conductor, an electric field is established. This field exerts a force on the free electrons, causing them to drift slowly through the lattice in the direction opposite to the field. Although individual electrons move randomly at very high speeds due to thermal energy, the net drift of electrons constitutes the measurable electric current.
Positive metal ions remain stationary within the lattice, vibrating around fixed positions.
Electrons are the mobile charge carriers that enable conduction.
The direction of conventional current (from positive to negative terminal) is opposite to the direction of electron flow.
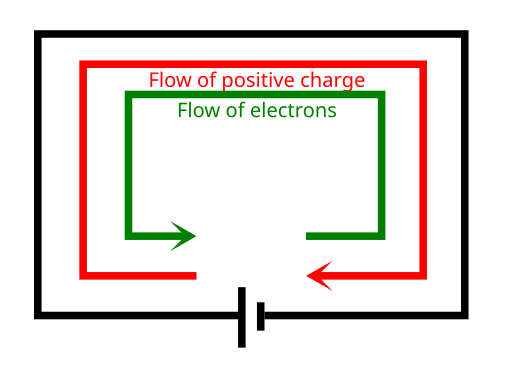
Diagram comparing conventional current (arrow from positive to negative) with electron flow (arrow from negative to positive) in a DC circuit. In metals, electrons are the actual charge carriers, so their drift direction opposes the conventional current direction. The figure focuses solely on direction, aligning with the syllabus note. Source.
Metals are excellent electrical conductors because they contain a very high number density of free electrons, allowing significant charge flow even with a small drift velocity. The effectiveness of conduction also depends on temperature; as temperature increases, lattice vibrations intensify, which can impede electron movement and increase resistance.
Charge Carriers in Electrolytes
An electrolyte is a substance that conducts electricity through the movement of ions when molten or dissolved in water. In this case, current is carried not by electrons but by positive (cations) and negative (anions) ions that move through the liquid or solution under the influence of an applied electric field.
Electrolyte: A liquid or solution containing ions that can conduct electricity through their movement.
When electrodes are inserted into an electrolyte and connected to a power source, an electric field is established in the liquid. This field causes:
Cations (positively charged ions) to move towards the cathode (negative electrode).
Anions (negatively charged ions) to move towards the anode (positive electrode).
The simultaneous movement of oppositely charged ions constitutes the electric current through the electrolyte. Each type of ion contributes to the total current according to its charge magnitude, concentration, and mobility.
Ion: An atom or molecule that has gained or lost one or more electrons, giving it an electric charge.
Electrolytic conduction differs from metallic conduction in several ways:
The charge carriers are ions rather than electrons.
Chemical changes occur at the electrodes, such as oxidation and reduction reactions, as ions gain or lose electrons.
The conductivity depends strongly on ion concentration and temperature.
The medium (usually a liquid or solution) allows ions to move freely, unlike the rigid lattice in metals.
Comparison: Charge Carriers in Metals and Electrolytes
1. Nature of Charge Carriers
Metals: Charge is carried by delocalised electrons, which are negatively charged.
Electrolytes: Charge is carried by ions; both positive and negative ions contribute to conduction.
2. Type of Conduction
Metallic conduction is purely electronic, involving no chemical change.
Electrolytic conduction is ionic, often accompanied by electrochemical reactions at electrodes.
3. Direction of Flow
In metals, electrons flow from negative to positive, whereas conventional current is defined as flowing from positive to negative.
In electrolytes, cations move towards the cathode and anions towards the anode, so current results from the combined motion of both types of ions.
4. Dependence on Physical Conditions
Metallic conductivity decreases with rising temperature due to increased lattice vibrations.
Electrolytic conductivity increases with temperature, as ion mobility and dissociation rise.
These differences highlight the distinct physical mechanisms underlying electrical conduction in solids and liquids.
Microscopic and Macroscopic Perspectives
On a microscopic scale, both electrons in metals and ions in electrolytes move randomly between collisions, but an applied electric field produces a net drift velocity that gives rise to a steady current. The magnitude of current depends on the number of available charge carriers and their mobility.
EQUATION
—-----------------------------------------------------------------
Current (I) = Charge (Q) ÷ Time (t)
I = Current in amperes (A)
Q = Charge in coulombs (C)
t = Time in seconds (s)
—-----------------------------------------------------------------
In both systems, current continuity is maintained — the charge that flows into any region must equal the charge that flows out, ensuring conservation of charge.
Summary of Key Points
Electric current results from the motion of charge carriers.
In metals, conduction is due to free electrons within a metallic lattice.
In electrolytes, conduction arises from the motion of positive and negative ions.
Conventional current direction is defined opposite to the direction of electron flow.
Conduction in metals and electrolytes follows distinct physical and chemical principles but both rely on the movement of charge to transfer energy efficiently.
FAQ
In metals, atoms are arranged in a lattice where outer electrons are loosely bound. These outer shell (valence) electrons become delocalised, forming a shared pool that can move freely throughout the lattice.
The positive metal ions remain fixed in position, while delocalised electrons move under an applied potential difference. Because only electrons are free to move, they are the sole charge carriers in metals.
Temperature affects their motion—higher temperatures increase ion vibrations, leading to greater resistance and reduced electron mobility.
Impurities introduce irregularities in the metallic lattice, disrupting the regular arrangement of ions.
These imperfections scatter delocalised electrons, increasing resistance.
Alloying (mixing metals) can deliberately increase resistance by reducing electron mean free path.
Very pure metals have fewer scattering centres, allowing more efficient conduction.
This explains why copper and silver, both with few lattice defects, are used for high-conductivity wiring.
No, ions move at different speeds depending on their mass, charge, and the medium’s viscosity.
Lighter ions (e.g. H⁺) tend to move faster than heavier ones (e.g. Cu²⁺).
Highly charged ions experience stronger electric forces but may interact more with surrounding solvent molecules, reducing mobility.
Temperature increases the average speed of ions by lowering viscosity and increasing kinetic energy.
Therefore, total current in an electrolyte results from the combined movement of cations and anions, each contributing differently to conduction.
In solids, ions are locked into a rigid lattice and cannot move freely. When an ionic compound melts or dissolves in water, the ions become mobile.
Only when ions are free can they respond to an electric field and move toward oppositely charged electrodes, allowing current to flow.
This is why molten salts and aqueous ionic solutions conduct electricity, whereas solid ionic compounds do not.
Electric current is defined as the net movement of charge. In electrolytes, both cations and anions move, but their motions combine to produce one overall current direction.
Cations (positive) move toward the cathode, transferring positive charge forward.
Anions (negative) move toward the anode, effectively transferring negative charge backward.
These two movements are equivalent in effect — together they maintain a single, consistent direction of current through the electrolyte, from the positive electrode to the negative electrode in conventional terms.
Practice Questions
Question 1 (2 marks)
Explain the difference between the charge carriers in a metal and in an electrolyte.
Mark Scheme:
1 mark for identifying electrons as the charge carriers in a metal.
1 mark for identifying ions (positive and negative) as the charge carriers in an electrolyte.
Question 2 (5 marks)
Describe and explain how electric current is conducted in both metals and electrolytes. In your answer, refer to the type and motion of the charge carriers in each case, and the effect of an applied potential difference.
Mark Scheme:
1 mark: States that in metals, conduction is due to delocalised electrons moving through a lattice of positive ions.
1 mark: Explains that when a potential difference is applied, the electrons drift towards the positive terminal (opposite to conventional current).
1 mark: States that in electrolytes, conduction occurs through the movement of ions.
1 mark: Explains that cations move towards the cathode and anions move towards the anode under the influence of an electric field.
1 mark: Compares or links the two systems (for example, noting that in metals the charge carriers are electrons while in electrolytes they are ions, and that only in electrolytes do chemical changes occur at the electrodes).

