The shapes of molecules and their bonding behaviors are explained through VSEPR theory and hybridization, both essential concepts for understanding molecular structure in biology.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory, which stands for Valence Shell Electron Pair Repulsion, is a model used to predict the three-dimensional geometry of molecules. It is based on the idea that electron pairs repel each other due to their negative charges, and will therefore arrange themselves as far apart as possible around a central atom to minimize repulsive forces. This arrangement determines the geometry of the molecule, which in turn affects its physical and chemical properties, including polarity, reactivity, and intermolecular interactions.
Key Concepts of VSEPR
The geometry of a molecule is dictated by the number of regions of electron density (bonding pairs and lone pairs) around the central atom.
The basic idea is that electron pairs will adopt an arrangement that minimizes electron-electron repulsion, leading to specific molecular shapes.
Bonding pairs are shared between atoms and contribute to the molecule’s framework.
Lone pairs (non-bonding pairs) occupy more space than bonding pairs due to their higher electron density, which leads to greater repulsion and often distorts bond angles.
Electron Domains and Geometries
Each region of electron density (whether a bonding pair, a double/triple bond, or a lone pair) is referred to as an electron domain. The number of electron domains around a central atom determines its electron domain geometry.
2 electron domains: Linear geometry, bond angle of 180 degrees.
3 electron domains: Trigonal planar geometry, bond angle of 120 degrees.
4 electron domains: Tetrahedral geometry, bond angle of 109.5 degrees.
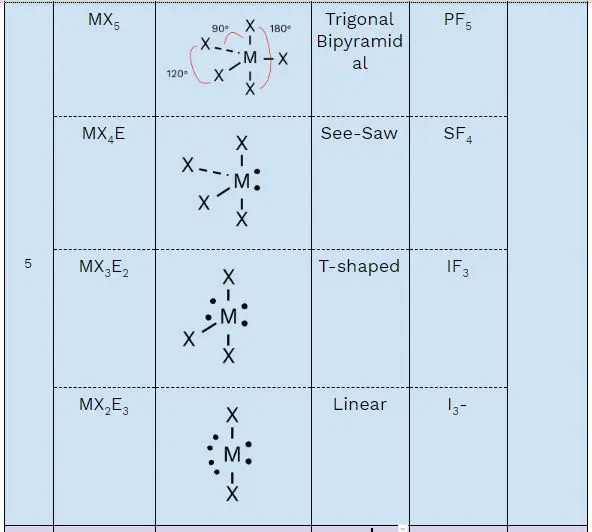
5 electron domains: Trigonal bipyramidal geometry, bond angles of 90 and 120 degrees.
6 electron domains: Octahedral geometry, bond angles of 90 degrees.
Note: Multiple bonds (double or triple) still count as one electron domain.
Molecular Geometry and Lone Pairs
While electron domain geometry includes all regions of electron density, molecular geometry only considers the positions of atoms. When there are lone pairs, they affect the geometry by compressing bond angles.
Examples:
AX₂: Linear, like carbon dioxide (CO₂)
AX₃: Trigonal planar, like boron trifluoride (BF₃)
AX₂E: Bent, like sulfur dioxide (SO₂)
AX₄: Tetrahedral, like methane (CH₄)
AX₃E: Trigonal pyramidal, like ammonia (NH₃)
AX₂E₂: Bent, like water (H₂O)
AX₅: Trigonal bipyramidal, like phosphorus pentachloride (PCl₅)
AX₄E: Seesaw, like sulfur tetrafluoride (SF₄)
AX₃E₂: T-shaped, like chlorine trifluoride (ClF₃)
AX₂E₃: Linear, like xenon difluoride (XeF₂)
AX₆: Octahedral, like sulfur hexafluoride (SF₆)
AX₅E: Square pyramidal, like bromine pentafluoride (BrF₅)
AX₄E₂: Square planar, like xenon tetrafluoride (XeF₄)



Bond Angles and Molecular Distortion
The presence of lone pairs causes greater repulsion than bonding pairs, which leads to compressed bond angles:
In NH₃, the lone pair compresses the H-N-H angle to about 107 degrees.
In H₂O, the two lone pairs compress the H-O-H angle further to about 104.5 degrees.
This distortion from ideal angles is essential to predict real molecular geometries and their behaviors.
Sigma and Pi Bonds
Chemical bonding involves orbital overlap, leading to the formation of sigma (σ) and pi (π) bonds. These bonds differ in strength, orientation, and how they affect molecular shape and behavior.
Sigma (σ) Bonds
Sigma bonds are formed by direct (end-to-end) overlap of orbitals along the internuclear axis.
They are the first bond formed between two atoms and are generally stronger than pi bonds.
All single bonds are sigma bonds.
Hybridized orbitals (such as sp, sp², or sp³) typically form sigma bonds.
Pi (π) Bonds
Pi bonds are formed by the sideways overlap of unhybridized p orbitals.
They are found in addition to a sigma bond in double or triple bonds.
Pi bonds are weaker due to less effective overlap.
Bond Compositions
Single bond = 1 σ bond
Double bond = 1 σ bond + 1 π bond
Triple bond = 1 σ bond + 2 π bonds
Effects of Pi Bonds
The greater the number of pi bonds, the shorter and stronger the bond becomes.
For instance, a triple bond (as in nitrogen gas, N₂) has a shorter bond length and higher bond energy than a double or single bond.
Example Breakdown

Image Courtesy of BC Open textbooks
Example 1: A molecule with 1 triple bond and 2 single bonds
Triple bond = 1 σ + 2 π
Each single bond = 1 σ
Total = 3 σ and 2 π bonds
Example 2: A molecule with 3 double bonds and 9 single bonds
Double bonds = 3 σ + 3 π
Single bonds = 9 σ
Total = 12 σ and 3 π bonds
Orbital Hybridization
Hybridization is a concept that explains the observed shapes and bonding behavior of molecules by considering the mixing of atomic orbitals.
What Is Hybridization?
Hybridization involves the mixing of atomic orbitals (like s and p orbitals) on the same atom to form new orbitals called hybrid orbitals.
These hybrid orbitals are identical in energy and shape and are arranged to minimize electron repulsion.
Hybridization allows atoms to form the correct number of bonds and explains bond angles predicted by VSEPR.
Types of Hybridization
sp³ Hybridization
Occurs when one s orbital mixes with three p orbitals.
Forms four sp³ hybrid orbitals.
Arrangement: Tetrahedral, with bond angles close to 109.5 degrees.
Example: CH₄ (methane) – four sigma bonds between carbon and hydrogen.
sp² Hybridization
Involves one s orbital and two p orbitals.
Forms three sp² hybrid orbitals and one unhybridized p orbital.
Arrangement: Trigonal planar, with 120-degree bond angles.
Example: C₂H₄ (ethylene) – double bond consists of one sigma bond (sp²) and one pi bond (p orbital overlap).
sp Hybridization
Combines one s orbital with one p orbital.
Forms two sp hybrid orbitals and two unhybridized p orbitals.
Arrangement: Linear, with 180-degree bond angles.
Example: C₂H₂ (ethyne) – triple bond with one sigma (sp) and two pi bonds (p orbitals).
sp³d and sp³d² Hybridization
Used for atoms that expand their octet, involving d orbitals:
sp³d: Five hybrid orbitals – trigonal bipyramidal geometry (like PCl₅).
sp³d²: Six hybrid orbitals – octahedral geometry (like SF₆).

Importance of Hybridization
Hybridization accounts for molecular shapes that cannot be explained by atomic orbitals alone. It allows:
Formation of the correct number of bonds.
Prediction of bond strength, length, and geometry.
Understanding of molecular reactivity.
Example: Carbon’s 2s and 2p orbitals combine to form four equivalent sp³ orbitals in methane (CH₄), allowing it to form four equivalent sigma bonds.
AP Free-Response Questions and VSEPR/Hybridization
AP questions often require you to draw Lewis structures, predict molecular geometries, and identify hybridization. Here are examples from past exams.
2007 Free Response
(a) Draw the Lewis structure of IF₃:
Central iodine atom bonded to three fluorines.
Two lone pairs on iodine.
(b) Predict the geometry:
T-shaped molecular geometry due to two lone pairs.
(c) For SO₂:
Both S-O bonds have the same length due to resonance.
Resonance creates equal bond character between the two oxygens.
(d) Hybridization of sulfur in SO₂:
sp² hybridization – three electron regions around sulfur.
2010 Free Response
(a) Draw the Lewis structure for ethyne (C₂H₂):
Each carbon triple bonded to each other and single bonded to one hydrogen.
(b) Identify the molecule with the shortest C-C bond:
Ethyne has the shortest due to triple bond (1 σ + 2 π).
(c) Given ethanoic acid:
Carbon x (double bonded to O): Trigonal planar – sp² hybridization.
Carbon y (single bonds): Tetrahedral – sp³ hybridization.

Essential Terms to Know
Bond Energy: Energy needed to break a bond.
Bond Length: Distance between nuclei of bonded atoms.
Coulombic Repulsion: Repulsion between like charges (negative-negative).
Lewis Structure: Diagram showing bonding and lone pairs.
Molecular Geometry: 3D shape of a molecule.
Pi Bond: Side-to-side overlap of unhybridized orbitals.
Sigma Bond: Direct overlap of orbitals along internuclear axis.
Valence Bond Theory: Bonds form by overlapping atomic orbitals.
sp Hybridization: Linear, 180-degree bond angles.
sp² Hybridization: Trigonal planar, 120-degree angles.
sp³ Hybridization: Tetrahedral, 109.5-degree angles.
sp³d Hybridization: Trigonal bipyramidal, expanded octet.
sp³d² Hybridization: Octahedral, six bonding regions.
VSEPR Theory: Electron pairs repel and determine geometry.
FAQ
Lone pairs influence both the geometry and hybridization of a molecule by occupying more space around the central atom than bonding pairs, which leads to geometric distortion. However, they also affect which orbitals are used in hybridization. In molecules like ammonia (NH₃), nitrogen is sp³ hybridized with one of the hybrid orbitals containing a lone pair. In water (H₂O), oxygen uses two sp³ orbitals for lone pairs. These nonbonding electrons still occupy hybrid orbitals and alter the observable shape. While they don’t change the electron domain count, they reduce symmetry and create shapes like bent and trigonal pyramidal.
Lone pairs increase repulsion more than bonding pairs.
They contribute to the count of hybrid orbitals used.
They do not contribute to the visible molecular geometry but still shape it.
They reduce bond angles compared to the ideal geometry.
VSEPR theory focuses on regions of electron density, not specific bonding arrangements. Resonance structures are multiple Lewis diagrams used to represent a molecule where electrons are delocalized, meaning the actual structure is a blend (resonance hybrid). Despite the different resonance forms, the number of electron domains around the central atom stays the same, so the geometry doesn’t change.
VSEPR counts total regions of electron density, not specific bond types.
Resonance affects bond lengths and strengths, but not overall geometry.
Example: In ozone (O₃), both bonds between oxygen atoms are equivalent due to resonance, but the central atom still has 3 electron domains → bent geometry.
Always base geometry on one complete resonance form and electron domains.
Expanded octets occur when a central atom forms more than four bonds, accommodating more than eight electrons. This happens in elements in the third period or beyond because they have d orbitals available for bonding. In terms of hybridization, these atoms utilize sp³d or sp³d² hybrid orbitals to form five or six bonding regions.
Examples include phosphorus (P), sulfur (S), chlorine (Cl), and xenon (Xe).
PCl₅ uses sp³d hybridization to make five sigma bonds.
SF₆ uses sp³d² hybridization to make six bonds in an octahedral shape.
These atoms expand their valence shell into empty d orbitals.
Yes, a molecule can contain multiple types of hybridization if it has more than one central atom or if atoms are in different bonding environments. Each atom hybridizes based on the number of regions of electron density around it.
In ethanoic acid (CH₃COOH), two carbon atoms have different hybridizations:
The methyl carbon (CH₃) is sp³ hybridized (four sigma bonds).
The carbon in the carboxyl group is sp² hybridized (three regions of electron density: one double bond and two single bonds).
Hybridization is determined atom by atom, based on local geometry and bonding.
A molecule's overall geometry may include tetrahedral, trigonal planar, and linear sections depending on the atoms involved.
Sigma bonds are stronger because they involve direct orbital overlap along the internuclear axis, allowing maximum electron density between nuclei. Pi bonds result from sideways overlap of p orbitals, which is less efficient, making them more exposed and easier to break. This affects reactivity and stability in significant ways:
Molecules with pi bonds (double or triple bonds) are generally more reactive.
Single bonds (sigma only) are more stable and harder to break.
In organic chemistry, pi bonds are often sites of reactivity in electrophilic addition reactions.
The presence of pi bonds can also affect molecular flexibility, since rotation around double/triple bonds is restricted.
Practice Questions
Predict the molecular geometry and hybridization of the central atom in phosphorus pentachloride (PCl₅). Explain your reasoning based on VSEPR theory and hybrid orbital concepts.
The molecular geometry of phosphorus pentachloride is trigonal bipyramidal. According to VSEPR theory, the central phosphorus atom forms five bonding pairs with five chlorine atoms and has no lone pairs. These five regions of electron density arrange themselves to minimize repulsion, leading to a trigonal bipyramidal shape. This geometry consists of three atoms in an equatorial plane and two in axial positions. The hybridization of phosphorus in this molecule is sp³d, which results from the mixing of one s orbital, three p orbitals, and one d orbital to form five equivalent sp³d hybrid orbitals for bonding.
Ethene (C₂H₄) contains a carbon-carbon double bond. Identify the type of bonds formed between the carbon atoms, explain the geometry around each carbon, and determine the hybridization of each carbon atom.
In ethene, each carbon atom forms three sigma bonds: two with hydrogen atoms and one with the other carbon. The double bond consists of one sigma bond formed by sp² hybrid orbitals and one pi bond formed by unhybridized p orbitals. Each carbon is surrounded by three regions of electron density, resulting in a trigonal planar geometry with 120-degree bond angles. Therefore, each carbon is sp² hybridized, having three sp² orbitals for sigma bonding and one unhybridized p orbital that overlaps sideways to form the pi bond in the double bond between the carbon atoms.

