IB Syllabus focus:
‘As biomass transfers through trophic levels, entropy increases. Living systems maintain low internal entropy by exporting disorder, largely via cellular respiration.’
Energy flow through ecosystems follows the laws of thermodynamics. The second law of thermodynamics explains why energy transfers are inefficient, entropy rises, and ecosystems rely on continuous energy input.
The Second Law of Thermodynamics
The second law of thermodynamics states that in any energy transfer or transformation, the total entropy of a system and its surroundings increases.
Entropy: A measure of disorder or randomness in a system.
In ecosystems, this principle explains why only a fraction of energy moves between trophic levels and why ecosystems depend on constant solar energy input.
Entropy in Biomass Transfers
When energy flows from producers to consumers, part of it is lost as heat through processes like respiration. This increases entropy, reducing usable energy.
Producers capture light energy and transform it into chemical energy via photosynthesis.
Consumers ingest and assimilate this energy, but respiration dissipates much of it as heat.
Decomposers recycle nutrients but also release energy as heat.
Thus, each trophic level transfer increases entropy and reduces the amount of energy available to higher levels.
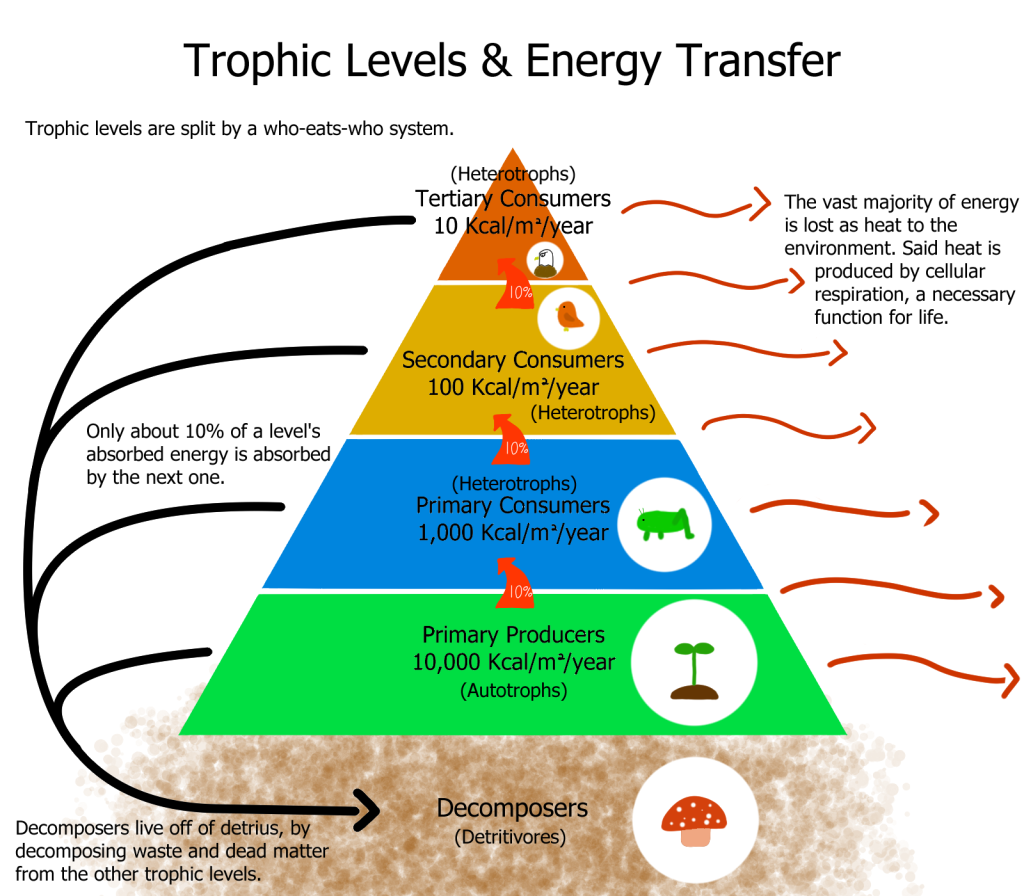
A labelled pyramid shows energy transfer between producers and consumers with explicit arrows indicating energy lost as heat via cellular respiration. This visually anchors the second law’s implication for ecosystems: entropy rises as usable energy degrades to heat at each transfer. (The ‘10%’ figures are illustrative; efficiencies vary.) Source.
Living Systems and Low Entropy
Living organisms maintain low internal entropy by creating ordered structures such as cells, tissues, and organs. This is possible because they continuously export disorder.
Cellular Respiration: The metabolic process by which glucose is broken down, releasing energy in the form of ATP and heat.
Through respiration, organisms release energy and export entropy to their surroundings.
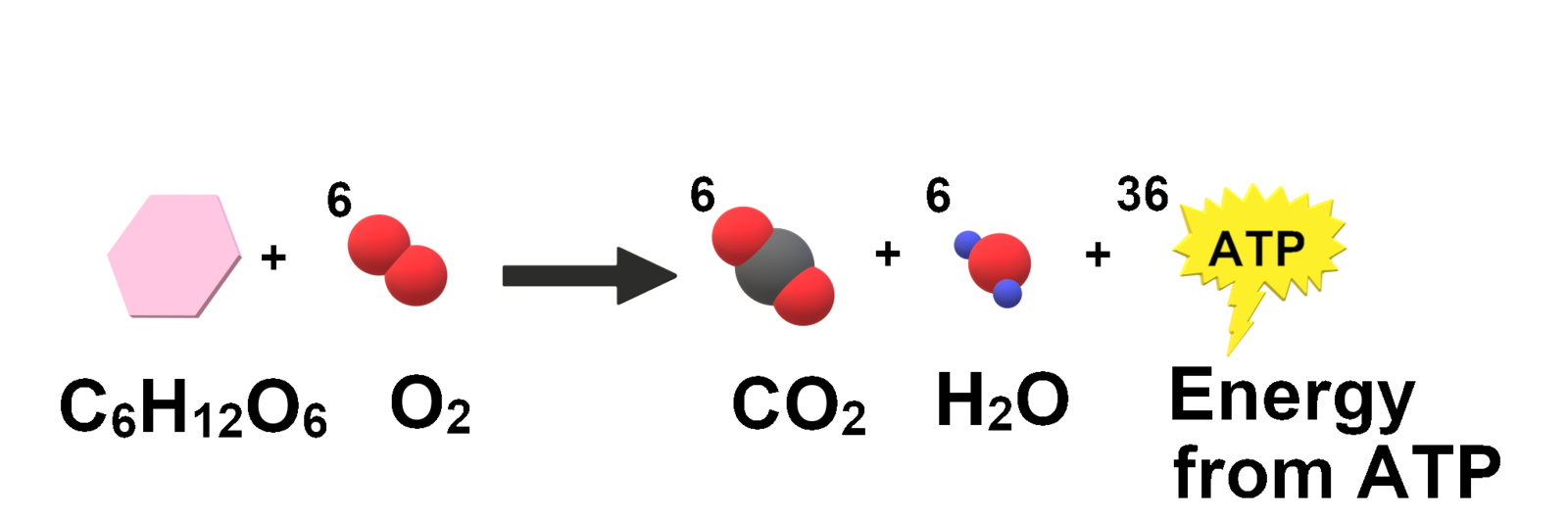
A simplified, clearly labelled equation for aerobic cellular respiration (glucose + oxygen → carbon dioxide + water + ATP). It highlights the biochemical pathway underlying entropy export; heat release during ATP production and use is implied though not shown explicitly. (Includes stoichiometric detail beyond the minimum needed for this subsubtopic.) Source.
Energy Flow and the Need for Input
Because entropy always increases, ecosystems require constant external energy input to remain functional. For Earth’s ecosystems, the primary source is the Sun.
Key points:
Without solar energy, entropy would rise unchecked, leading to collapse.
The energy pyramid narrows at higher trophic levels because energy availability decreases with each transfer.
Entropy explains the limited number of trophic levels in most ecosystems.
Entropy and Food Chains
In food chains:
Producers fix solar energy into biomass.
Primary consumers assimilate part of this biomass, exporting entropy via respiration.
Secondary and tertiary consumers transfer progressively less energy.
This process highlights that energy transfers are inefficient. Only a small proportion (often around 10%) passes upward, while entropy accumulates at each step.
Heat as a Manifestation of Entropy
Most energy loss in ecosystems occurs as heat, a direct reflection of the second law. Heat is energy in a highly disordered state, unsuitable for biological work.
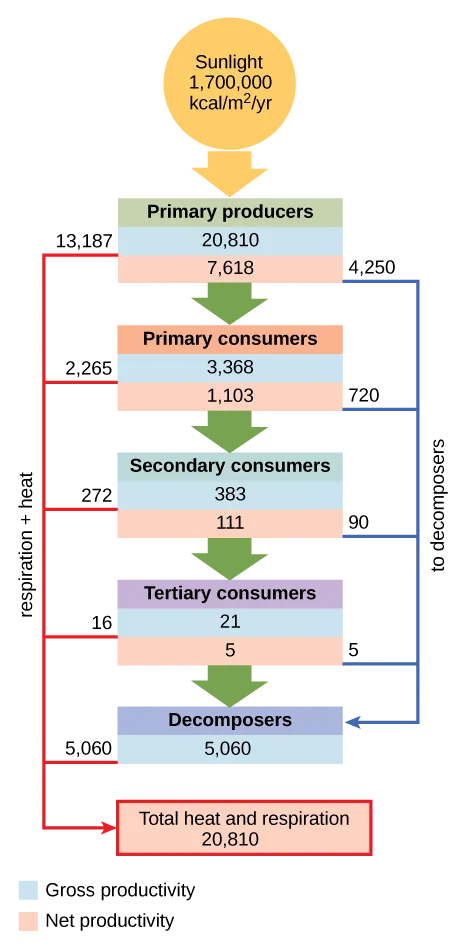
Conceptual diagram of the Silver Springs ecosystem quantifying energy flow and the cumulative losses as metabolic heat at each trophic level. It demonstrates why energy pyramids are upright and why ecosystems require continual external energy input as entropy increases. (Includes numerical details and decomposer flows beyond the minimum required by this subsubtopic.) Source.
Herbivory, predation, and metabolism all generate heat.
This exported heat contributes to the entropy of the biosphere.
Heat cannot be recycled, so ecosystems must rely on new solar energy input daily.
Ecosystem-Level Implications
Entropy shapes the dynamics of ecosystems in several ways:
Energy degradation: Each transfer moves energy from concentrated, usable forms (chemical bonds) to dispersed, less useful forms (heat).
Trophic inefficiency: Fewer organisms can be supported at higher trophic levels because of energy loss.
System openness: Ecosystems are open systems, relying on solar input to counteract the natural drift towards disorder.
Exporting Disorder in Ecosystems
Ecosystems as a whole maintain structure by exporting disorder. Key mechanisms include:
Respiration: Releases heat and carbon dioxide, raising environmental entropy.
Excretion: Waste products are expelled, reducing internal disorder.
Decomposition: Dead matter is broken down, releasing heat and nutrients.
Together, these processes ensure that internal order is preserved even as total entropy rises.
Entropy, Ecosystem Stability, and Limits
Entropy also explains why ecosystems have carrying capacities and why energy-intensive food webs are limited:
As entropy increases, less energy remains available for productive growth.
High entropy makes ecosystems more vulnerable to disruption without constant energy inflow.
Biodiversity enhances stability by distributing energy flow, but entropy still dictates ultimate limits.
Key Takeaways for IB ESS
The second law of thermodynamics governs energy flow in ecosystems.
Entropy always increases during biomass transfers, reflected in heat loss and inefficiency.
Living systems maintain order by exporting disorder through respiration and other processes.
Ecosystems require continuous solar input to offset entropy and sustain life.
FAQ
As energy is lost as heat at each transfer, less usable energy remains to support organisms at higher levels.
The result is a natural restriction: food chains rarely exceed four or five trophic levels. Any additional level would not gain enough energy to sustain a viable population.
Heat energy disperses randomly into the surroundings and cannot be converted back into biomass.
Unlike nutrients, which cycle, heat dissipates irreversibly. This one-way flow explains why continuous solar input is essential to counter entropy.
Ecological efficiency measures how much energy passes from one trophic level to the next.
Higher entropy means lower efficiency.
Processes like respiration and digestion release heat, increasing disorder.
Variation in entropy explains why efficiency differs between ecosystems and species.
As entropy increases, energy becomes more dispersed, reducing availability for higher-level consumers.
Complex food webs can buffer this loss because multiple pathways distribute energy more widely, making the system more resilient to disturbances.
Respiration breaks down glucose into ATP, but much of the energy is lost as heat.
This shows entropy in action: while cells maintain order by producing usable energy, they export disorder to the environment. This balance allows life to persist while increasing universal entropy.
Practice Questions
Question 1 (2 marks):
Explain why energy transfers between trophic levels are inefficient according to the second law of thermodynamics.
Mark scheme:
1 mark: States that energy is lost as heat during processes such as respiration.
1 mark: Links this to the second law of thermodynamics by stating that entropy (disorder) increases with each energy transfer.
Question 2 (5 marks):
Discuss how the second law of thermodynamics explains the need for continuous energy input into ecosystems.
Mark scheme:
1 mark: States that energy transfers in ecosystems are inefficient due to entropy increase.
1 mark: Notes that much of the energy is lost as heat at each trophic level.
1 mark: Explains that this heat cannot be recycled into usable energy for organisms.
1 mark: Identifies the Sun as the main continuous external energy source for ecosystems.
1 mark: Makes the link that without constant input, ecosystems would collapse as energy degrades into unusable forms.

