OCR Specification focus:
‘Explain phosphodiester bond formation and breakdown; describe ATP and ADP structures.’
Nucleotides form the foundation of all nucleic acids, linking through condensation reactions to create polynucleotides. Energy-carrying molecules such as ATP and ADP are also nucleotides.
Polynucleotide Formation
Structure of a Nucleotide
A nucleotide is a biological molecule made up of three main components:
A pentose sugar (deoxyribose in DNA, ribose in RNA)
A phosphate group
A nitrogenous base (adenine, thymine, guanine, cytosine, or uracil)
The combination of these components allows nucleotides to link together to form long chains, known as polynucleotides.
Nucleotide: The monomer unit of nucleic acids, consisting of a phosphate group, a pentose sugar, and a nitrogenous base.
Formation of Phosphodiester Bonds
Nucleotides are joined by condensation reactions between the phosphate group of one nucleotide and the hydroxyl group (–OH) on the carbon-3 (C3’) of the pentose sugar in another. This reaction releases a molecule of water and forms a phosphodiester bond, resulting in a sugar-phosphate backbone that gives the polynucleotide structural stability.
The phosphodiester bond connects carbon-5 (C5’) of one nucleotide’s sugar to carbon-3 (C3’) of the next.
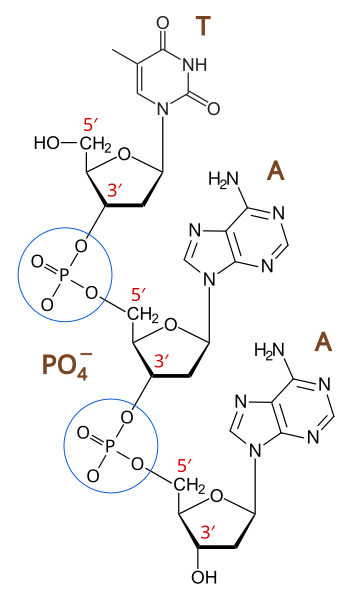
Labeled diagram of a 3′–5′ phosphodiester bond linking nucleotides into a sugar-phosphate backbone. The 5′ phosphate of one nucleotide joins the 3′ hydroxyl of the next, establishing strand directionality. This directly illustrates the condensation linkage discussed in polynucleotide formation. Source
Each chain therefore has directionality, with one free phosphate group at the 5' end and one free hydroxyl group at the 3' end.
Phosphodiester bond: The covalent bond between the phosphate group of one nucleotide and the hydroxyl group on the C3’ carbon of another nucleotide.
This directional bonding is crucial because it allows replication and transcription enzymes to recognize strand orientation.
Breakdown of Phosphodiester Bonds
Phosphodiester bonds can be broken through hydrolysis reactions, the reverse of condensation. Hydrolysis adds water back to the bond, separating individual nucleotides and allowing nucleic acids to be degraded during cellular recycling or replication errors.
Hydrolytic enzymes, such as nucleases, catalyze this breakdown.
The process is vital for nucleotide turnover, DNA repair, and RNA degradation.
The Role of ATP and ADP as Phosphorylated Nucleotides
The Structure of ATP and ADP
Adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are examples of phosphorylated nucleotides, meaning they contain one or more phosphate groups added to the nucleotide structure.
ATP consists of adenine (a nitrogenous base), ribose (a pentose sugar), and three phosphate groups.
ADP is structurally similar but contains only two phosphate groups.
The bonds between phosphate groups are high-energy bonds, specifically phosphoanhydride bonds, which release large amounts of energy when broken.
Phosphorylated nucleotide: A nucleotide containing one or more phosphate groups bonded to its sugar component, e.g. ATP or ADP.
ATP as the Universal Energy Currency
ATP acts as the primary energy carrier within cells. Energy released from metabolic reactions, such as respiration, is used to phosphorylate ADP to ATP. This energy can later be released by hydrolyzing ATP back to ADP and inorganic phosphate (Pi).
ATP synthesis occurs via condensation, catalyzed by ATP synthase, during processes like oxidative phosphorylation and photophosphorylation.
ATP breakdown (hydrolysis) is catalyzed by ATPase, releasing energy for cellular work.
EQUATION
—-----------------------------------------------------------------
ATP Hydrolysis = ATP → ADP + Pi + energy
ATP = Adenosine triphosphate
ADP = Adenosine diphosphate
Pi = Inorganic phosphate
—-----------------------------------------------------------------
This process is reversible, allowing the cell to recycle ATP molecules continuously.
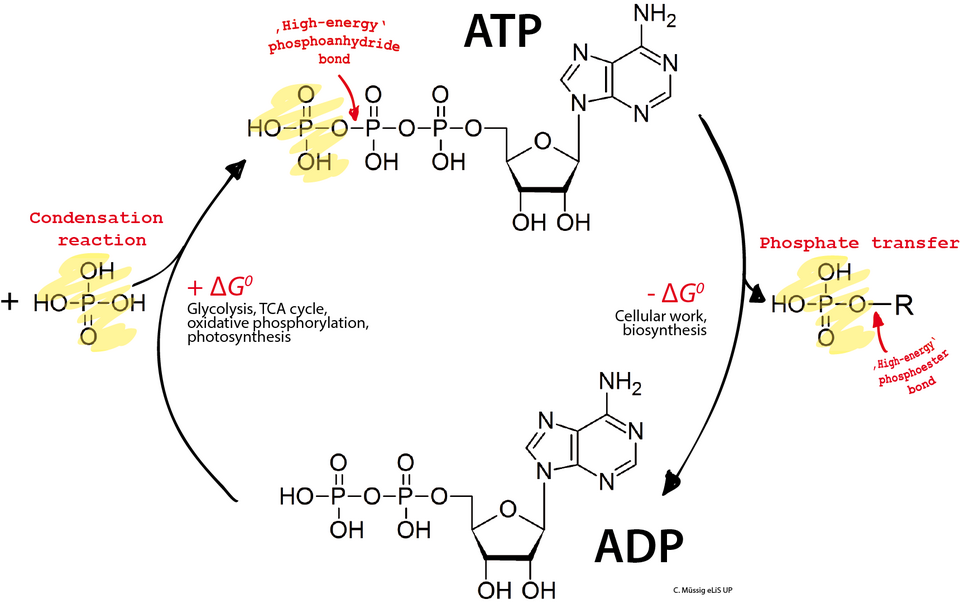
Diagram of the ATP ↔ ADP + Pi cycle highlighting hydrolysis (energy release) and re-phosphorylation (energy input). This aligns with ATP’s role as the cell’s recyclable energy currency. The image also names related species (AMP, PPi, Mg²⁺); these are acceptable extras but not required by the syllabus. Source
Energy Release and Storage
The hydrolysis of ATP releases around 30.5 kJ mol⁻¹ of energy, used for active transport, muscle contraction, and biosynthesis.
The phosphate bonds are not inherently “high-energy”; instead, the energy comes from the instability of these bonds and the stabilization of products (ADP and Pi) after hydrolysis.
ATP (Adenosine triphosphate): A phosphorylated nucleotide that serves as the main energy currency in cells, releasing energy when hydrolyzed to ADP and inorganic phosphate.
Following ATP hydrolysis, the released inorganic phosphate can be transferred to another molecule in a process known as phosphorylation, which often activates enzymes or changes molecular shape to initiate metabolic reactions.
Linking ATP and Polynucleotide Chemistry
Shared Features Between ATP and Nucleotides
Both ATP and the nucleotides that form DNA or RNA share fundamental chemical features:
Each contains a pentose sugar, phosphate group(s), and a nitrogenous base.
In both, condensation reactions link phosphate and sugar groups.
Energy from ATP hydrolysis is often required for the polymerization of nucleotides during nucleic acid synthesis.
This biochemical link underscores how energy transfer and genetic information storage share a common molecular origin in the chemistry of nucleotides.
Relevance of Phosphodiester Bonds in ATP Function
Although ATP’s energy release primarily involves the breaking of phosphoanhydride bonds, the formation of phosphodiester bonds is fundamental in connecting ATP’s ribose sugar to its adenine base and the first phosphate group. This illustrates how phosphodiester chemistry underpins both information molecules (like DNA) and energy molecules (like ATP).
Summary of Key Processes
Condensation reactions join nucleotides via phosphodiester bonds, forming polynucleotide chains.
Hydrolysis breaks these bonds, releasing individual nucleotides.
ATP and ADP are phosphorylated nucleotides central to cellular energy transfer.
ATP hydrolysis provides energy for metabolic reactions, while ATP synthesis stores energy from respiration and photosynthesis.
The shared chemical principles between nucleotides and ATP demonstrate the unity of molecular biology in energy and information systems.
FAQ
The phosphodiester bond is highly stable due to its covalent nature and resistance to hydrolysis under physiological conditions. The negative charge on the phosphate group repels nucleophilic attack, reducing spontaneous bond cleavage.
In DNA, the sugar-phosphate backbone is shielded within the double helix, further protecting the bond from enzymatic degradation. However, specific enzymes such as nucleases can break these bonds during controlled cellular processes like DNA replication and repair.
ATP contains ribose (not deoxyribose) and has three phosphate groups instead of one. It also lacks a nitrogenous base that pairs with another molecule, meaning it does not form part of a nucleic acid chain.
Key differences:
ATP’s phosphates are linked by high-energy phosphoanhydride bonds.
The extra phosphates give ATP its energy-storage function.
DNA and RNA nucleotides, in contrast, primarily serve as information carriers rather than energy molecules.
ATP can release energy rapidly and in small, manageable amounts suitable for most cellular reactions. Its hydrolysis to ADP and Pi is a single-step reaction catalysed by ATPase.
Efficiency arises because:
ATP is water-soluble and diffuses easily within the cytoplasm.
It can be regenerated quickly by phosphorylation of ADP.
The energy change per mole (~30.5 kJ) matches most biological requirements, avoiding energy waste.
Magnesium ions (Mg²⁺) act as essential cofactors for enzymes that use or synthesise ATP. They bind to ATP’s phosphate groups, stabilising the molecule’s negative charges.
This stabilisation:
Helps enzymes such as ATP synthase or kinases correctly position ATP for catalysis.
Reduces repulsion between phosphate groups, making hydrolysis or phosphorylation more efficient.
Without Mg²⁺, ATP would be less reactive and many energy-dependent reactions would slow dramatically.
RNA is less stable than DNA because its ribose sugar has an extra hydroxyl (–OH) group at the 2′ carbon. This group can act as a nucleophile and attack the phosphodiester bond, promoting self-cleavage under alkaline conditions.
In DNA, the deoxyribose lacks this 2′ hydroxyl group, so the backbone is more chemically stable.
This difference explains why DNA is the long-term genetic store, while RNA is suited for short-term information transfer.
Practice Questions
Question 1 (2 marks)
Describe how a phosphodiester bond is formed between two nucleotides in a DNA molecule.
Mark scheme:
(1 mark) Condensation reaction occurs between the phosphate group of one nucleotide and the hydroxyl group on the 3′ carbon of the sugar of another.
(1 mark) Formation of a covalent phosphodiester bond and release of a molecule of water (H₂O).
Question 2 (5 marks)
Explain how the structure of ATP allows it to act as an immediate source of energy in the cell and how it is regenerated from ADP.
Mark scheme:
(1 mark) ATP consists of adenine, ribose, and three phosphate groups.
(1 mark) Energy is stored in the bonds between phosphate groups (phosphoanhydride bonds).
(1 mark) When ATP is hydrolysed to ADP and inorganic phosphate (Pi), energy is released for cellular processes.
(1 mark) ATP hydrolysis is catalysed by ATPase and provides energy for reactions such as active transport and muscle contraction.
(1 mark) ATP is regenerated from ADP and Pi by condensation during respiration or photosynthesis, catalysed by ATP synthase.

