OCR Specification focus:
‘Describe the electron transport chain, proton gradients and ATP synthase in oxidative phosphorylation and photophosphorylation.’
The chemiosmotic theory explains how energy stored in proton gradients drives ATP synthesis during oxidative phosphorylation and photophosphorylation, uniting processes in respiration and photosynthesis through a shared biochemical mechanism.
The Basis of Chemiosmosis
The chemiosmotic theory, proposed by Peter Mitchell in 1961, revolutionised understanding of energy transduction. It describes how electron transport through membranes generates a proton gradient, which powers ATP synthesis via ATP synthase.
Chemiosmosis occurs in two major contexts in biology:
Oxidative phosphorylation in mitochondria during aerobic respiration.
Photophosphorylation in chloroplasts during photosynthesis.
Although the energy source differs — chemical energy from reduced coenzymes in mitochondria and light energy in chloroplasts — the mechanism is fundamentally the same: both rely on proton gradients across membranes.
The Electron Transport Chain (ETC)
Structure and Location
In mitochondria, the electron transport chain (ETC) is embedded within the inner mitochondrial membrane, folded into cristae to increase surface area. The chain comprises a series of electron carrier proteins arranged in a precise sequence of redox centres.
Key Components
Complex I (NADH dehydrogenase): Accepts electrons from reduced NAD (NADH).
Complex II (succinate dehydrogenase): Accepts electrons from reduced FAD (FADH₂).
Ubiquinone (Coenzyme Q): Transfers electrons between Complex I/II and Complex III.
Complex III (cytochrome bc₁ complex): Passes electrons to cytochrome c.
Cytochrome c: A small mobile carrier transferring electrons to Complex IV.
Complex IV (cytochrome c oxidase): Transfers electrons to oxygen, forming water.
Oxygen acts as the final electron acceptor, maintaining the flow of electrons through the chain.
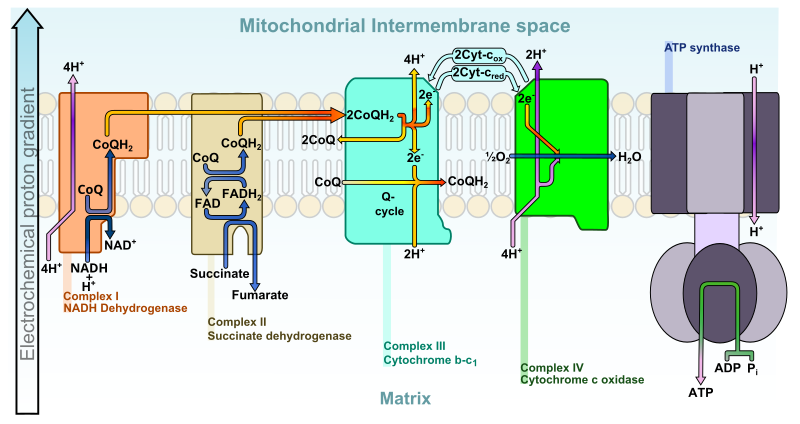
Diagram of the mitochondrial electron transport chain, showing Complexes I–IV, ubiquinone, cytochrome c, and ATP synthase. Electrons move to oxygen while energy from redox reactions pumps protons into the intermembrane space, establishing the electrochemical gradient that drives ATP synthesis. This figure focuses on oxidative phosphorylation without extraneous pathways. Source.
Electron Transport Chain (ETC): A sequence of membrane-bound proteins that transfer electrons through redox reactions, coupling this to proton pumping across a membrane.
Each electron transfer releases a small quantity of energy, sufficient to drive active transport of protons (H⁺ ions) from the mitochondrial matrix into the intermembrane space.
Formation of the Proton Gradient
Proton Pumping
The energy released by electron transfers through Complexes I, III, and IV powers proton pumps, actively transporting H⁺ ions across the inner mitochondrial membrane. This creates two gradients:
A chemical gradient, as proton concentration increases in the intermembrane space.
An electrical gradient, as the matrix becomes relatively negative.
Together, these form an electrochemical gradient, or proton motive force (PMF).
Proton Motive Force (PMF): The combined effect of chemical and electrical gradients of protons across a membrane, used to drive ATP synthesis.
Because the inner mitochondrial membrane is impermeable to protons, they can only re-enter via specialised channels — the ATP synthase complexes.
ATP Synthase and Chemiosmotic Coupling
Structure of ATP Synthase
ATP synthase is a large multi-subunit enzyme complex composed of two main parts:
F₀ subunit: Embedded in the membrane, forming a proton channel.
F₁ subunit: Extends into the matrix and catalyses the synthesis of ATP.
As protons flow down their electrochemical gradient through the F₀ subunit, they cause rotation of the enzyme’s internal shaft, inducing conformational changes in the F₁ subunit that enable ADP and inorganic phosphate (Pi) to combine into ATP.
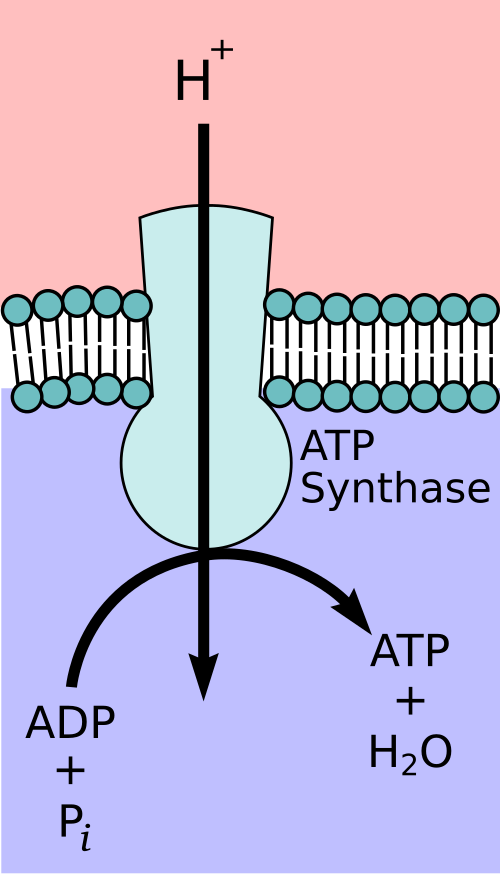
Schematic of F₀F₁-ATP synthase embedded in a membrane, with F₀ forming the proton channel and F₁ catalysing ATP formation. Proton flow through F₀ drives rotation of the central shaft, producing conformational changes in F₁ that couple proton motive force to phosphorylation of ADP. The diagram focuses on core subunits relevant to OCR and omits advanced subunit detail. Source.
ATP Synthase: An enzyme complex that utilises the energy of a proton gradient to catalyse the formation of ATP from ADP and Pi.
This process of chemiosmotic coupling ensures that energy released from redox reactions is efficiently converted into a high-energy phosphate bond in ATP.
Oxidative Phosphorylation in Context
Integration with Respiration
The oxidative phosphorylation stage follows the Krebs cycle, using the reduced coenzymes NADH and FADH₂ generated in earlier stages. These donate electrons to the ETC, linking substrate-level reactions to oxidative energy yield.
Each molecule of NADH typically yields around 2.5 ATP, while FADH₂ yields about 1.5 ATP, due to differing entry points into the chain and numbers of protons pumped.
EQUATION
—-----------------------------------------------------------------
Overall Aerobic Respiration Reaction:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ~38 ATP
C₆H₁₂O₆ = Glucose (substrate)
O₂ = Final electron acceptor
CO₂ = Waste product
ATP = Energy carrier (adenosine triphosphate)
—-----------------------------------------------------------------
The precise ATP yield can vary depending on organism and efficiency of transport processes.
Photophosphorylation and Comparison
In chloroplasts, light energy excites electrons in photosystem II, which are passed along an ETC in the thylakoid membrane. Energy from electron transfer drives proton pumping into the thylakoid lumen, establishing a proton gradient similar to that in mitochondria.
When protons diffuse back through ATP synthase into the stroma, ATP is formed — this is known as photophosphorylation.
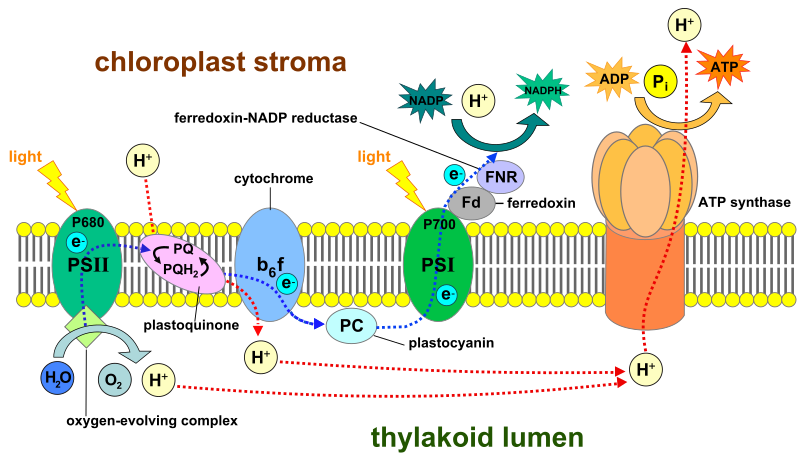
Labeled diagram of the thylakoid membrane showing light-driven electron flow, proton pumping, and ATP synthesis via ATP synthase. It emphasises the proton gradient in the lumen that powers photophosphorylation. Extra detail includes NADPH production and full photosystem components to place chemiosmosis in photosynthesis. Source.
The process supports the Calvin cycle in the light-independent stage of photosynthesis.
Although the energy source differs — light versus chemical oxidation — both rely on the same chemiosmotic principles.
The Universal Nature of Chemiosmosis
Chemiosmosis is a universal mechanism for ATP production in living organisms. Its features include:
Membrane-bound electron carriers forming an ETC.
Proton gradients across selective membranes.
ATP synthase complexes using proton motive force.
Coupling of redox energy to phosphorylation of ADP.
The theory elegantly unites diverse energy-conversion systems, demonstrating that both respiration and photosynthesis depend on proton-driven ATP synthesis. This principle underlies nearly all bioenergetic processes, from mitochondria in animal cells to chloroplasts in plants and even bacterial plasma membranes.
FAQ
Peter Mitchell’s chemiosmotic theory was initially controversial but later supported by strong experimental evidence.
When the inner mitochondrial membrane is disrupted, ATP synthesis stops, showing that the proton gradient is essential.
Artificial proton gradients across membranes can directly drive ATP production even without electron transport.
Uncoupling agents, like DNP, dissipate the proton gradient and prevent ATP formation despite ongoing electron flow, confirming the gradient’s role in energy coupling.
These results collectively demonstrated that ATP synthesis depends on the electrochemical gradient, not a high-energy chemical intermediate.
Uncoupling proteins allow protons to re-enter the mitochondrial matrix without passing through ATP synthase, bypassing ATP production.
This releases the stored energy from the proton motive force as heat rather than chemical energy.
In mammals, UCP1 (thermogenin) in brown adipose tissue helps maintain body temperature, especially in newborns and hibernating animals.
The process is called non-shivering thermogenesis, where energy is used for warmth instead of ATP generation.
The difference lies in where electrons enter the electron transport chain.
NADH donates electrons to Complex I, which pumps protons at Complexes I, III, and IV.
FADH₂, however, donates electrons directly to Complex II, bypassing Complex I.
Because fewer protons are pumped, FADH₂ contributes to a smaller proton gradient, yielding about 1.5 ATP, compared with 2.5 ATP per NADH molecule.
Protons flow through the F₀ channel in ATP synthase, binding to sites on a rotating c-ring.
Each proton binding causes a mechanical shift that rotates the ring and the central γ-subunit.
This rotation induces conformational changes in the F₁ catalytic sites, sequentially binding ADP and Pi, synthesising ATP, and releasing it.
This remarkable mechanism converts electrochemical energy into mechanical rotation, and then into chemical bond energy.
Yes — bacteria and archaea also use chemiosmosis to generate ATP.
Instead of mitochondria, they use their plasma membrane as the site of the electron transport chain and ATP synthase.
Protons are pumped into the periplasmic space (or outside the cell), creating a proton motive force across the cell membrane.
In some anaerobic bacteria, alternative electron acceptors like nitrate or sulphate replace oxygen.
The underlying principle is the same: proton gradients power ATP synthesis, though the structure and location differ from eukaryotic systems.
Practice Questions
Question 1 (2 marks)
Explain the role of the proton gradient in oxidative phosphorylation.
Mark Scheme:
1 mark: States that the proton gradient provides energy for ATP synthesis.
1 mark: Describes that protons diffuse through ATP synthase, driving the conversion of ADP and inorganic phosphate (Pi) into ATP.
Question 2 (5 marks)
Describe how the chemiosmotic theory explains the production of ATP in mitochondria.
Mark Scheme:
Award up to 5 marks for the following points:
1 mark: Electrons from reduced NAD and FAD pass along the electron transport chain in the inner mitochondrial membrane.
1 mark: Energy released from electron transfer is used to pump protons (H⁺) from the matrix into the intermembrane space.
1 mark: This creates a proton gradient and an electrochemical potential difference across the membrane.
1 mark: Protons diffuse back into the matrix through ATP synthase, down their electrochemical gradient.
1 mark: The flow of protons provides energy to phosphorylate ADP, forming ATP.
Allow alternative wording such as “proton motive force” for the proton gradient and “chemiosmotic coupling” for the process linking electron transport to ATP synthesis.

