OCR Specification focus:
‘Identify NH4+ and cations Cu2+, Fe2+, Fe3+, Mn2+, Cr3+ using test-tube reactions linked to precipitation behaviour.’
A range of simple aqueous reactions allows reliable identification of ammonium ions and several transition-metal cations. These qualitative tests depend on characteristic gas evolution and distinctive precipitate colours.
Tests for Ammonium and Transition-Metal Cations
Ammonium ion, NH4+
The ammonium ion is identified through its reaction with warm aqueous alkali to release ammonia gas, a pungent, alkaline gas detectable by its effect on moist indicator paper.
Ammonia (NH3): A colourless, alkaline gas formed when ammonium ions react with hydroxide ions.
A key point is that no visible precipitate forms during this test, making gas detection essential.
Procedure for detecting NH4+
Add aqueous NaOH to the test solution.
Warm gently without boiling.
Test any gas evolved using moist red litmus paper.
A colour change to blue confirms NH3 and therefore NH4+.
This diagram illustrates the confirmatory test for ammonium ions, where warming with aqueous sodium hydroxide releases ammonia gas that turns damp red litmus paper blue. Source
Transition-metal cations and precipitation behaviour
Transition-metal cations form characteristic hydroxide precipitates when aqueous hydroxide ions are added. These precipitates differ in colour, oxidation behaviour and solubility, providing a set of diagnostic observations.
Precipitate: A solid formed when ions in solution combine to produce an insoluble compound.
Each metal cation forms a distinctive precipitate with OH− and may undergo further changes upon standing or on addition of excess reagents.
Tests with aqueous hydroxide, OH−
Aqueous sodium hydroxide or aqueous ammonia may be used as the source of hydroxide ions. The colours listed below apply immediately after addition and, where relevant, after exposure to air.
Copper(II) ion, Cu2+
Add a few drops of NaOH(aq) or NH3(aq).
A blue precipitate of Cu(OH)2 forms.
With excess NH3(aq), the precipitate dissolves to give a deep blue solution, indicating formation of a soluble complex.

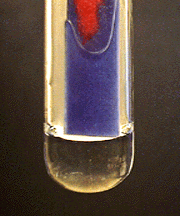
The image shows copper(II) hydroxide, Cu(OH)₂, appearing as a blue precipitate when hydroxide ions react with Cu²⁺ in solution. Source
Iron(II) ion, Fe2+
Addition of OH− forms a green precipitate of Fe(OH)2.
On standing in air, the precipitate oxidises to a brown species as Fe2+ converts to Fe3+.
Iron(III) ion, Fe3+
Addition of OH− yields a brown precipitate of Fe(OH)3, a key identifying feature.
The precipitate does not readily dissolve in excess reagent.

This photo shows iron(III) hydroxide, Fe(OH)₃, forming as an orange-brown precipitate when hydroxide ions are added to a solution containing Fe³⁺. Source
Manganese(II) ion, Mn2+
A pale pink to light brown precipitate of Mn(OH)2 forms.
Rapid oxidation in air produces a darker brown solid, aiding identification.
Chromium(III) ion, Cr3+
Add OH− to obtain a grey-green precipitate of Cr(OH)3.
In excess NaOH(aq), the precipitate dissolves to form a dark green solution, showing formation of a soluble chromite complex.
A normal sentence is included here to maintain continuity before introducing any further definition blocks.
Complex ion: A species formed when a central metal ion binds to surrounding ligands through coordinate bonds.
Distinguishing reactions using aqueous ammonia, NH3(aq)
Some transition-metal cations behave differently with aqueous ammonia than with sodium hydroxide, offering additional diagnostic power.
Cu2+: Produces a blue precipitate, dissolving in excess to give a deep blue complex.
Fe2+ and Fe3+: Form green and brown precipitates respectively; neither dissolves significantly in excess ammonia.
Mn2+: Gives a light brown precipitate with no substantial dissolution.
Cr3+: Forms a grey-green precipitate which dissolves in excess NH3(aq) to give a purple complex, though observations may vary with concentration.
Practical testing notes
When performing these qualitative tests, consistent reagent volumes and dropwise addition are essential. This ensures that observations such as colour intensity, dissolution, or oxidation effects can be interpreted clearly.
Key practical reminders:
Use fresh solutions of Fe2+ and Mn2+ to avoid pre-oxidation.
Observe precipitates both immediately and after standing, as oxidation is part of the diagnostic behaviour for several ions.
Warm gently when testing for NH4+, as excessive heating may release ammonia too rapidly.
Use moist litmus or pH indicator paper; dry paper will not react with gaseous ammonia.
Summary of characteristic observations (non-tabulated)
NH4+: Releases NH3 on warming with alkali; turns moist red litmus blue.
Cu2+: Blue precipitate; dissolves in excess NH3(aq) to give deep blue solution.
Fe2+: Green precipitate, browns on standing due to oxidation.
Fe3+: Brown precipitate.
Mn2+: Light brown precipitate, darkens on oxidation.
Cr3+: Grey-green precipitate, dissolves in excess OH− or NH3 to give green or purple complexes.
These observations provide a systematic method for identifying ammonium and key transition-metal cations required by the OCR A-Level Chemistry specification.
FAQ
Ammonia is only alkaline when dissolved in water, not in its dry gaseous form. Damp litmus paper provides the moisture needed for ammonia to dissolve and produce hydroxide ions.
Without moisture, the gas will not cause a colour change, even if ammonia is present. This can lead to false negative results during ammonium testing.
Certain metal ions, such as Fe2+ and Mn2+, are readily oxidised by oxygen in the air. This oxidation changes the oxidation state of the metal ion.
As a result, the hydroxide precipitate formed initially can convert into a different compound with a new colour, providing useful diagnostic information during qualitative analysis.
Aqueous ammonia acts as both a weak base and a ligand. In excess ammonia, ligand exchange occurs, forming a soluble deep blue copper–ammonia complex.
Sodium hydroxide does not act as a ligand in the same way, so copper(II) hydroxide remains insoluble in excess hydroxide ions.
Fe2+ and Mn2+ ions are easily oxidised by oxygen in air, even before any reagents are added. If solutions are left standing, partial oxidation may already have occurred.
This can alter precipitate colours or produce mixed observations, making it harder to correctly identify the ion present.
Adding reagents slowly allows clear observation of the initial precipitate before excess reagent affects the result. Some ions form precipitates that dissolve in excess reagent.
Dropwise addition ensures that both formation and dissolution stages can be observed, improving the reliability of the identification process.
Practice Questions
A student adds aqueous sodium hydroxide to an unknown solution and warms the mixture. A gas is evolved which turns damp red litmus paper blue.
a) Identify the ion present in the solution.
b) Name the gas produced.
(2 marks)
Identifies ammonium ion, NH4+ (1 mark)
Names ammonia, NH3, as the gas produced (1 mark)
A solution contains one of the following ions: Cu2+, Fe2+, Fe3+ or Cr3+.
Describe how you could use aqueous sodium hydroxide and aqueous ammonia to identify the ion present. Your answer should include the observations you would make and how these lead to a conclusion.
(5 marks)
Award marks for any correct, logically linked set of observations and conclusions. Indicative content includes:
Addition of OH− produces a precipitate with a characteristic colour (1 mark)
Correct colour of precipitate stated for at least one ion:
Cu2+ gives a blue precipitate
Fe2+ gives a green precipitate (turns brown on standing)
Fe3+ gives a brown precipitate
Cr3+ gives a grey-green precipitate (1 mark)
Correct use of aqueous ammonia to distinguish ions (1 mark)
Cu2+ precipitate dissolves in excess NH3 to give a deep blue solution, or Cr3+ precipitate dissolves in excess reagent (1 mark)
Clear conclusion identifying the ion based on the stated observations (1 mark)
Maximum 5 marks.

