OCR Specification focus:
‘Substitute haloalkanes with excess ethanolic ammonia or amines to give primary, secondary, and tertiary amines.’
Introduction
Aliphatic amines can be synthesised efficiently from haloalkanes using nucleophilic substitution reactions, enabling progressive formation of primary, secondary, and tertiary amines under controlled conditions.
Making Aliphatic Amines from Haloalkanes
Overview of the Reaction Pathway
Haloalkanes are versatile synthetic starting materials because the carbon–halogen bond is polar, allowing bond substitution through nucleophilic attack. When treated with excess ethanolic ammonia, or alternatively with amines, the substitution reaction produces a series of aliphatic amines.
Amines are compounds derived from ammonia in which hydrogen atoms are replaced by alkyl groups.
Amine: A compound in which one or more hydrogen atoms of ammonia (NH₃) are replaced by alkyl groups.
This reaction pathway is essential in organic synthesis, providing access to nitrogen-containing functional groups that participate in further synthetic transformations.
After learning the principles, students should be able to outline how haloalkanes are progressively substituted to yield primary, secondary, and tertiary amines, and understand why controlling the reaction conditions is crucial.
Mechanism of Nucleophilic Substitution
The formation of aliphatic amines occurs by nucleophilic substitution (Sᴺ2) when a nucleophile donates a pair of electrons to an electron-deficient carbon.
Nucleophile: A species that donates an electron pair to form a new covalent bond with an electron-deficient atom.
This transformation is a nucleophilic substitution reaction, typically SN2 for primary haloalkanes.
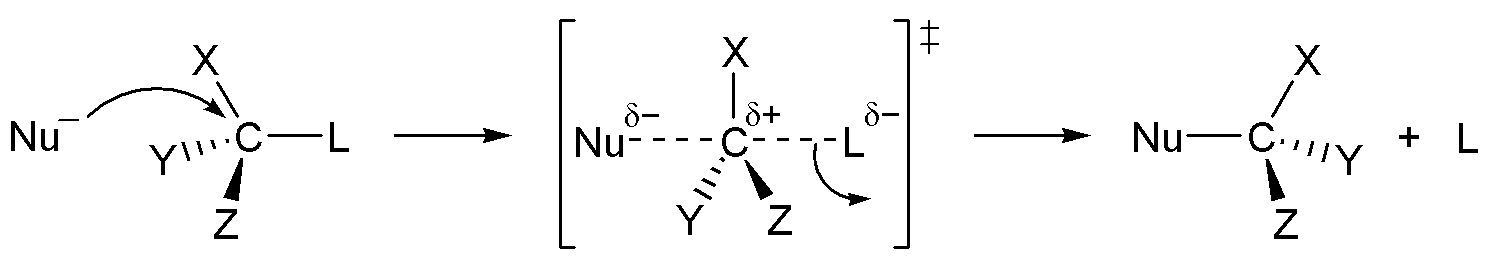
This diagram shows the key features of an SN2 substitution: the nucleophile forms a new C–N bond as the halide leaves in the same step. The reaction occurs by backside attack, so the carbon centre undergoes inversion of configuration when it is chiral. Source
Nucleophilic attack by ammonia or an amine displaces the halide ion. A normal explanatory sentence should always follow a definition, so here we emphasise that this is a one-step mechanism proceeding via a transition state, typical of haloalkanes that are primary.
Key features of the mechanism include:
Electron pair donation by ammonia or an amine to the δ⁺ carbon of the haloalkane.
Heterolytic fission of the C–X bond, releasing a halide ion (X⁻).
Proton transfer when ammonia is used, producing an amine and an ammonium halide salt.
Reaction carried out in ethanol solvent to prevent substitution by hydroxide ions present in water.
Formation of Primary Amines
When a haloalkane reacts with excess ethanolic ammonia, a primary amine is produced.
Process summary:
Ammonia acts as the nucleophile.
A primary alkylammonium salt forms immediately after substitution.
Deprotonation by excess ammonia forms the primary amine.
A halide ion is released, forming ammonium halide as a by-product.
Ammonia first forms an alkylammonium intermediate, which is then deprotonated to give the primary amine.
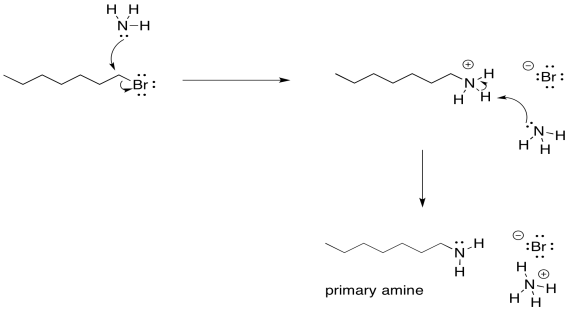
The haloalkane undergoes nucleophilic substitution with NH₃ to form an alkylammonium ion. A base, often another ammonia molecule, removes a proton to regenerate a neutral primary amine and an ammonium salt by-product. Source
Using excess ammonia drives formation towards primary amines by reducing the likelihood of further substitution.
Formation of Secondary and Tertiary Amines
Primary amines formed in the first step can themselves act as nucleophiles. Because they contain a nitrogen atom with a lone electron pair, they readily attack remaining haloalkane molecules.
This means that if ammonia is not in excess, the reaction continues:
Pathway for successive substitutions:
Primary amine + haloalkane → secondary amine (via nucleophilic substitution).
Secondary amine + haloalkane → tertiary amine.
Further substitution may also yield quaternary ammonium salts, where all four hydrogens originally from ammonia are replaced.
Important factors influencing product distribution:
Concentration of ammonia versus haloalkane.
Type of haloalkane, since tertiary haloalkanes undergo elimination rather than substitution.
Temperature and solvent, both affecting reaction rate and selectivity.
Preparing Secondary Amines Directly Using Amines
Instead of ammonia, an amine can be used as the nucleophile from the outset. This allows more controlled synthesis of secondary amines, especially when the target amine has two different alkyl groups.
Primary amine: An amine in which one hydrogen of ammonia has been replaced by an alkyl group.
A sentence must follow to meet the specification rules: primary amines act as effective nucleophiles because their alkyl groups increase electron density on nitrogen, strengthening the lone pair’s reactivity.
When a primary amine reacts with a haloalkane, the product is a secondary amine, subject to the same potential for further substitution if one alkyl group remains bonded to nitrogen.
Solvent and Reagent Conditions
Using ethanol rather than water as the solvent prevents hydrolysis, which would otherwise convert the haloalkane into an alcohol. Ethanolic ammonia must be used in excess to minimise polysubstitution.
Conditions typically include:
Warm, ethanolic solution
Sealed container to prevent ammonia evaporation
Excess ammonia for primary amine synthesis
Controlled ratios of amine and haloalkane for secondary and tertiary amine synthesis
Typical Reaction Pattern
Below is a generalised pattern for substitution with ammonia.
Overall sequence:
Haloalkane + ammonia → alkylammonium salt
Alkylammonium salt + ammonia → primary amine + ammonium halide
Primary amine + haloalkane → secondary amine
Secondary amine + haloalkane → tertiary amine
Tertiary amine + haloalkane → quaternary ammonium salt
Each step is governed by nucleophilicity and stoichiometric control.
Why Excess Ammonia Is Required
If ammonia is not used in excess, the initially formed primary amine will dominate as the nucleophile, resulting in higher proportions of secondary and tertiary amines. To obtain predominantly primary amines, ammonia must outcompete the amines formed during the reaction.
Using excess ethanolic ammonia helps to favour primary amine formation by reducing further alkylation to secondary and tertiary amines.

This figure illustrates how using a large excess of ammonia favours formation of the primary amine by reducing further alkylation. It also shows the contrasting outcome when haloalkane is in excess, which is additional detail beyond the core syllabus requirement. Source
Key Takeaways for OCR A-Level Chemistry
Excess ethanolic ammonia favours primary amine formation.
Amines can substitute further to produce secondary, tertiary, and quaternary ammonium products.
Nucleophilic substitution using ammonia or amines is essential for extending nitrogen-containing organic molecules in multi-step synthesis pathways.
FAQ
Tertiary haloalkanes are sterically hindered, meaning the carbon bonded to the halogen is shielded by bulky alkyl groups.
This prevents effective backside attack by ammonia or amines, which is required for SN2 substitution. As a result, elimination reactions often dominate instead of amine formation.
Ethanol dissolves both haloalkanes and ammonia while minimising competing reactions.
Water would introduce hydroxide ions, which act as nucleophiles and form alcohols rather than amines. Ethanol therefore improves selectivity for amine formation.
Ammonia is volatile and can escape easily if the reaction vessel is open.
Loss of ammonia reduces its concentration, increasing the likelihood of secondary and tertiary amine formation and lowering the yield of the desired primary amine.
Quaternary ammonium salts form when a tertiary amine undergoes further substitution.
They are ionic, often water-soluble, and cannot be converted into neutral amines by deprotonation, limiting their usefulness in further organic synthesis.
The rate depends on the strength of the carbon–halogen bond.
Iodoalkanes react fastest
Bromoalkanes react at a moderate rate
Chloroalkanes react more slowly
This trend reflects the increasing bond strength from C–I to C–Cl, affecting how easily the halide leaves during substitution.
Practice Questions
Bromoethane reacts with excess ethanolic ammonia to form an amine.
a) State the type of reaction that occurs.
b) Name the organic product formed.
(2 marks)
a)
Nucleophilic substitution (1 mark)
b)
Ethylamine (or ethanamine) (1 mark)
Chloropropane reacts with ammonia in ethanol to form an amine.
a) Describe the mechanism for the reaction between chloropropane and ammonia.
b) Explain why excess ammonia is used in this reaction rather than equimolar amounts.
(5 marks)
a) Mechanism description (3 marks)
Ammonia acts as a nucleophile, donating a lone pair to the carbon atom bonded to chlorine (1 mark)
Carbon–chlorine bond breaks to release Cl⁻ (1 mark)
Formation of an alkylammonium intermediate followed by deprotonation to give the amine (1 mark)
b) Use of excess ammonia (2 marks)
Reduces further substitution of the amine product to form secondary and tertiary amines (1 mark)
Increases likelihood of haloalkane reacting with ammonia rather than the amine product, favouring primary amine formation (1 mark)

