OCR Specification focus:
‘Amines act as bases: nitrogen lone pairs accept protons; they form salts with dilute acids.’
Amines display characteristic basic behaviour due to the lone pair on the nitrogen atom, enabling them to accept protons and form salts with suitable acids. This section explores the fundamental ideas behind amine basicity and the essential reactions involved in salt formation.
Basicity of Amines
Amines act as Brønsted–Lowry bases, meaning they accept protons (H⁺). This fundamental property arises from the presence of a lone pair of electrons on the nitrogen atom, which can form a dative covalent bond with a proton. In A-Level Chemistry, understanding basicity requires examining how readily an amine donates this lone pair, the factors affecting its availability, and how different types of amines respond in acid–base environments.
Amines act as bases because the nitrogen atom carries a lone pair of electrons that can accept a proton (H⁺) to form a new N–H bond.
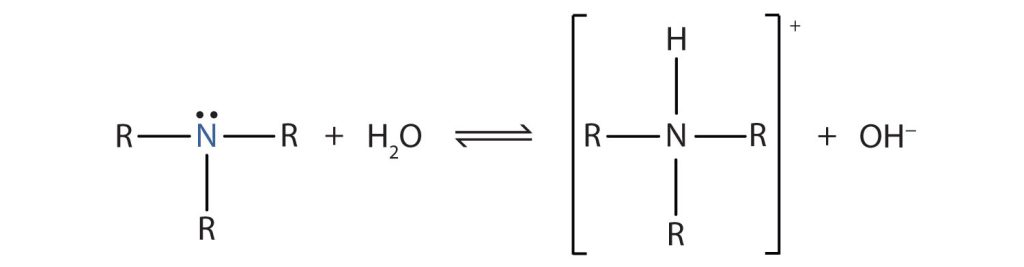
This diagram shows a generic amine using its nitrogen lone pair to accept a proton, forming a protonated ammonium ion and hydroxide ions. The positive charge on nitrogen highlights the basic nature of amines. Source
Brønsted–Lowry base: A species that accepts a proton (H⁺).
A key feature of amine basicity is the formation of alkylammonium ions or arylammonium ions. Once a proton is accepted, the amine becomes positively charged, altering its reactivity and solubility. Students often encounter questions involving comparing the basicity of ammonia, aliphatic amines, and aromatic amines, which demonstrates how structural environment influences nitrogen's lone pair availability.
Electron Pair Availability and Basic Strength
The availability of the nitrogen lone pair determines the basic strength of an amine. Factors that affect lone pair availability include:
Inductive effects from alkyl groups, which increase electron density on nitrogen and strengthen basicity.
Resonance effects in aromatic amines, where the lone pair overlaps with the benzene ring and becomes less available for protonation, decreasing basicity.
Solvent interactions, especially hydrogen bonding in aqueous solutions, which can stabilise protonated species and influence measured basic strengths.
Protonation of Amines
In aqueous or acidic conditions, amines readily undergo protonation, forming substituted ammonium ions. This behaviour is central to many separation and purification techniques in organic chemistry and underpins the formation of salts outlined in the OCR specification.
In aqueous conditions, a typical reaction is the protonation of an amine to form a substituted ammonium ion.
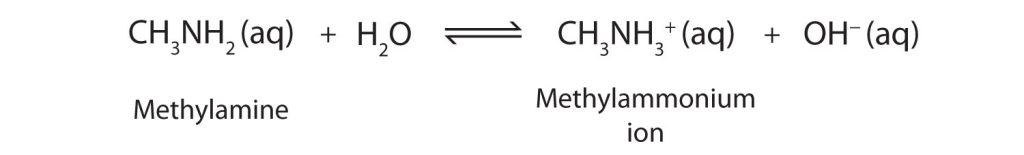
Methylamine accepts a proton to form the methylammonium ion, clearly illustrating the formation of a positively charged conjugate acid. This concrete example reinforces the general protonation behaviour of amines. Source
Protonation: The process in which a species gains a proton (H⁺).
After protonation, the resulting ammonium ion is more polar and often more soluble in water than the corresponding amine, which is especially important in synthetic and analytical contexts.
Salt Formation with Dilute Acids
Amines react with various dilute acids, such as hydrochloric acid or ethanoic acid, to form stable amine salts. These salts demonstrate ionic behaviour and are generally solid or readily soluble crystalline substances, depending on the acid involved.
Formation of Ammonium Salts
The overall process involves the lone pair on nitrogen accepting a proton from the acid, leading to ionic bond formation between the positively charged ammonium species and the negative counter-ion from the acid.
Reaction with dilute hydrochloric acid produces alkylammonium chloride or arylammonium chloride.
Reaction with dilute sulphuric acid produces alkylammonium sulphate or arylammonium sulphate.
Reaction with organic acids results in ammonium ethanoates or similar salts.
Aminium Salt Formation (General) = RNH₂ + H⁺ → RNH₃⁺
R = Alkyl or aryl group (no units)
H⁺ = Proton supplied by the acid (mol)
RNH₃⁺ = Ammonium ion formed (mol)
Salt formation not only indicates the base strength of the original amine but also provides a convenient way of transforming volatile or insoluble amines into more manageable ionic forms. These salts can be converted back to the parent amines by adding a strong base such as sodium hydroxide, which removes the proton and regenerates the free amine.
When an amine is protonated, the nitrogen becomes a positively charged ammonium centre, and an acid provides the counter-ion to form an amine salt.
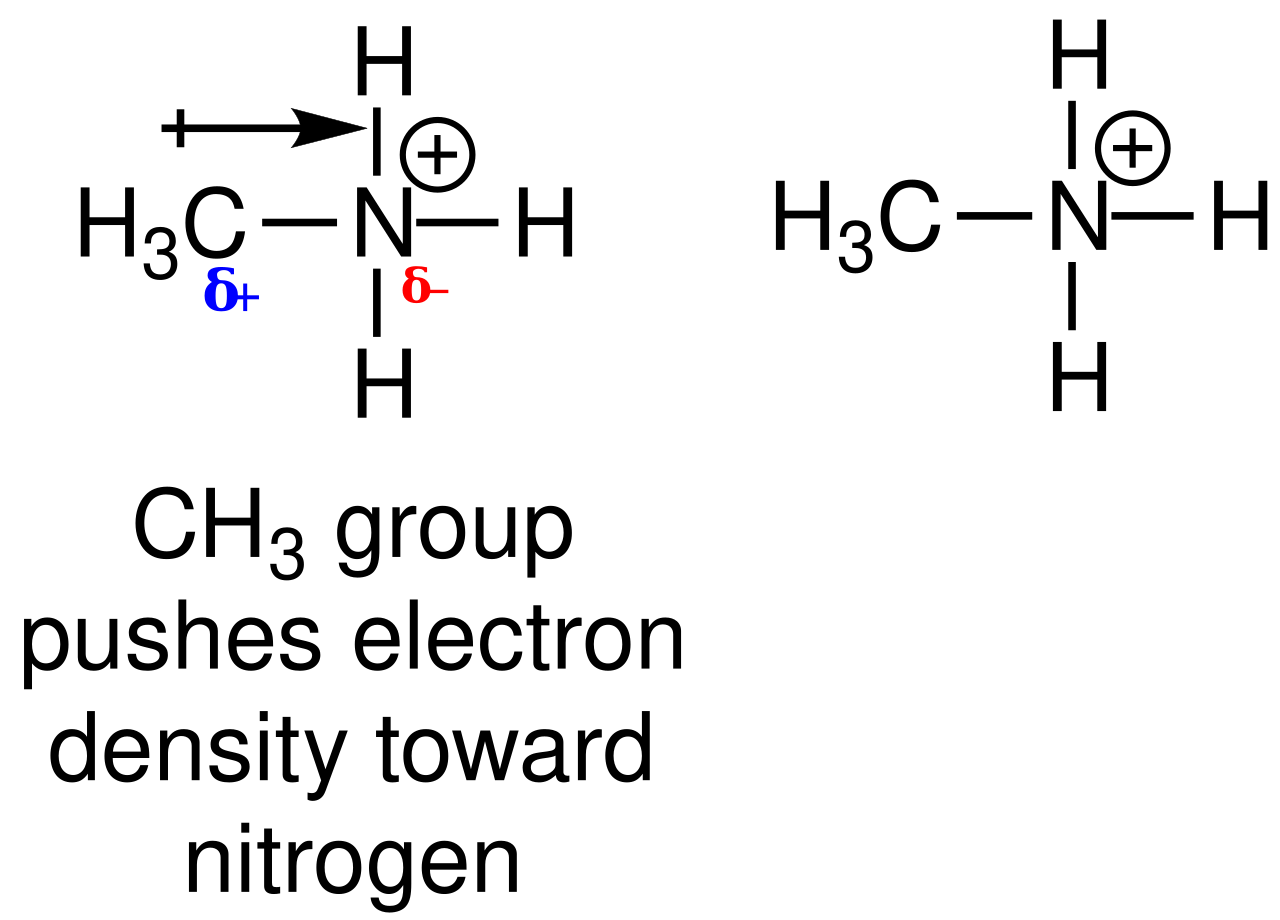
These Lewis structures highlight how nitrogen becomes four-bonded and positively charged after protonation. The diagram focuses on bonding and charge rather than the accompanying anion that completes an amine salt. Source
Solubility and Physical Consequences
Amines, especially higher-chain aliphatic amines, may exhibit limited solubility in water, but their salts are usually much more soluble due to strong ion–dipole interactions. This difference in solubility is frequently exploited in laboratory purification steps. Salt formation also often results in changes to melting point, crystalline structure, and volatility, reflecting the shift from a molecular to an ionic compound.
Relative Basicities of Amines
Although the specification emphasises the conceptual understanding of amines acting as bases, it is useful to consider the general trends in their basic strengths:
Aliphatic amines are typically stronger bases than ammonia, as alkyl groups donate electron density to nitrogen.
Aromatic amines such as phenylamine are weaker bases because resonance delocalisation reduces the availability of the nitrogen lone pair.
Ammonia serves as a baseline for comparison and helps students appreciate how substituents influence reactivity.
The interplay between inductive and resonance effects provides essential insight into predicting reactivity, acidity of conjugate acids, and behaviour of amines in multi-step synthetic routes.
Acid–Base Behaviour in Organic Contexts
Understanding the basicity and salt formation of amines is essential for organic synthesis, purification, and mechanism analysis. The protonation–deprotonation steps occur widely in reaction pathways and influence nucleophilicity, polarity, and compound stability. In many reaction schemes, chemists rely on amine basicity to direct transformations, stabilise intermediates, or enhance solubility during isolation.
Importance in Practical Chemistry
Organic chemists frequently use acid–base reactions to separate amines from neutral or acidic compounds. Amines can be selectively protonated, extracted into an aqueous phase, and regenerated later. Their ability to form salts with dilute acids underpins this widely used technique and supports many preparative and analytical processes encountered throughout A-Level organic chemistry.
FAQ
Amine salts are ionic compounds formed from positively charged ammonium ions and negatively charged counter-ions from acids.
Ionic attractions between these charged particles are much stronger than the intermolecular forces present in neutral amines. As a result, amine salts typically have higher melting points and exist as crystalline solids rather than volatile liquids or gases.
Many amines have strong, unpleasant odours because they are volatile molecules.
When an amine forms a salt:
It becomes ionic rather than molecular
Volatility decreases significantly
Odour is greatly reduced or eliminated
This is why amines are often stored or handled as their salts in laboratories.
Amine salts dissolve readily in water because they consist of charged ions.
Water molecules form strong ion–dipole interactions with both the ammonium ion and the counter-ion from the acid. Neutral amines rely mainly on hydrogen bonding and dipole–dipole forces, which are weaker and less effective for solvation.
Amine salts themselves are not basic in water because the nitrogen lone pair is no longer available after protonation.
However, if a strong base is added:
The proton can be removed from the ammonium ion
The original amine is regenerated
Basic behaviour is restored
This reversible behaviour is important in organic purification techniques.
Although aromatic amines are weaker bases, their nitrogen lone pair can still accept a proton under acidic conditions.
Resonance reduces lone pair availability but does not prevent protonation entirely. In the presence of dilute acids, aromatic amines form stable arylammonium salts, though less readily than aliphatic amines.
Practice Questions
Ethylamine reacts with dilute hydrochloric acid.
a) Explain why ethylamine acts as a base in this reaction.
b) Name the type of salt formed.
(2 marks)
a) Explanation of basicity (1 mark)
States that ethylamine has a lone pair of electrons on the nitrogen atom that can accept a proton (H⁺).
b) Identification of salt (1 mark)
Correctly identifies the salt as an alkylammonium salt or ethylammonium chloride.
A student compares the behaviour of ammonia, ethylamine, and phenylamine when each reacts with dilute hydrochloric acid.
a) Describe how each substance reacts with the acid.
b) Explain, in terms of structure and electron availability, why ethylamine is a stronger base than phenylamine.
(5 marks)
a) Description of reactions (3 marks total)
Ammonia accepts a proton to form the ammonium ion / ammonium chloride (1 mark).
Ethylamine accepts a proton to form an alkylammonium ion / ethylammonium chloride (1 mark).
Phenylamine accepts a proton to form an arylammonium ion / phenylammonium chloride (1 mark).
b) Explanation of difference in basicity (2 marks total)
Ethylamine is more basic because the alkyl group has a positive inductive effect that increases electron density on the nitrogen (1 mark).
Phenylamine is less basic because the nitrogen lone pair is delocalised into the benzene ring by resonance, making it less available to accept a proton (1 mark).

