OCR Specification focus:
‘Reduce nitroarenes using tin and concentrated hydrochloric acid to form aromatic amines.’
Aromatic amines are essential intermediates in organic synthesis, dyes, pharmaceuticals, and polymers. Understanding how to form them from nitroarenes is a core skill in A-Level Chemistry.
Making Aromatic Amines from Nitroarenes
Aromatic amines are formed by reducing nitroarenes such as nitrobenzene, converting the –NO₂ group into an –NH₂ group. This transformation is a key step in aromatic chemistry because it provides access to amine-functionalised aromatic compounds that can undergo further reactions, including acylation, diazotisation, and coupling.
When nitroarenes undergo reduction, they typically proceed through several intermediate stages before producing the final aromatic amine. In school-level chemistry, however, the focus is on the practical method described in the specification using tin (Sn) and concentrated hydrochloric acid (HCl).
Understanding the Nitro Group Reduction
The nitro group, –NO₂, is an electron-withdrawing substituent that strongly influences the reactivity of aromatic rings. Reducing it requires a suitable reducing agent capable of supplying electrons and hydrogen atoms.
Reduction: A chemical process involving the gain of electrons or decrease in oxidation state.
In the context of nitroarenes, reduction converts the nitrogen from a high oxidation state in the nitro group to a lower oxidation state in the amine group.
Nitroarene reduction using tin and concentrated hydrochloric acid is highly efficient, producing an amine salt that must then be regenerated into the free amine.
Reagents and Conditions
Aromatic amines are produced under reflux to ensure the reaction mixture is heated safely and consistently.
Key reagents include:
Tin (Sn) — the reducing metal.
Concentrated hydrochloric acid (HCl) — provides hydrogen ions and creates a strongly reducing environment.
A nitroarene, e.g. nitrobenzene.
An alkali, typically sodium hydroxide (NaOH), used after reduction to liberate the free aromatic amine.
The role of tin is to donate electrons, while acid supplies protons for stepwise reduction. Together, they convert the nitro group into an amine via complex intermediates.
Mechanistic Overview of Reduction
While the full mechanism involves several protonation and electron-transfer steps, A-Level Chemistry focuses on the overall reduction process. Recognising each stage supports better understanding of the conditions required.
The nitro group is reduced in stages:
Nitroarene → nitrosoarene
Nitrosoarene → hydroxylamine derivative
Hydroxylamine derivative → aromatic amine
Students need not memorise these intermediates for examination but should be aware that multi-stage reduction occurs.
Formation of the Amine Salt
When nitroarenes are reduced using tin and concentrated hydrochloric acid, the reaction initially produces an amine salt rather than the free amine.
Amine salt: An ionic compound formed when an amine accepts a proton from an acid, producing a positively charged ammonium species.
Aromatic amines behave as bases due to the lone pair on nitrogen, allowing them to accept protons from acids. In this reaction, the amine forms its hydrochloride salt (e.g. phenylammonium chloride).
After reduction, excess alkali is added to release the free amine, which is the desired product.
The reduction is typically carried out by heating the nitroarene with tin and concentrated hydrochloric acid under reflux.
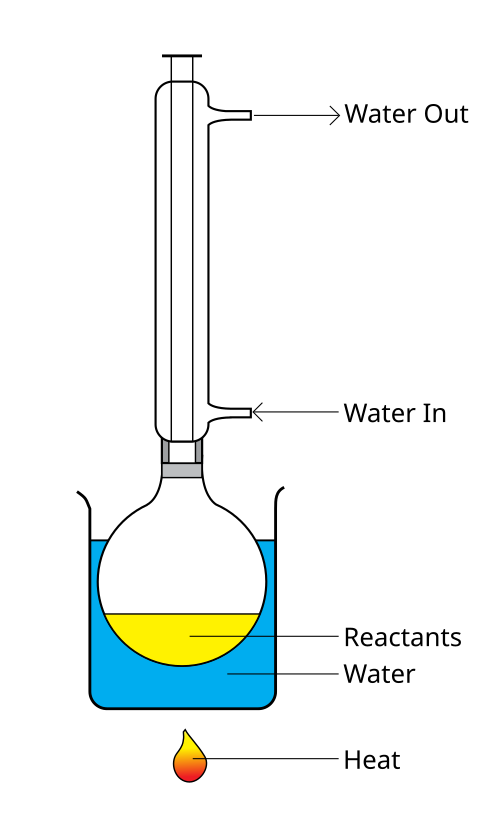
A reflux setup allows a reaction mixture to be heated for an extended time while preventing solvent loss. Vapours condense in the vertical condenser and return to the flask, keeping the reaction volume approximately constant. Source
Overall Reaction Pathway
The reduction and subsequent liberation of the amine may be summarised as:
Step 1: Reduction under acidic conditions
Nitroarene + Sn + concentrated HCl
→ Aromatic amine salt + SnCl₂
Step 2: Addition of alkali after reduction
Amine salt + NaOH
→ Free aromatic amine + NaCl + H₂O
Under the strongly acidic conditions, the product is first formed as an anilinium (arylammonium) chloride salt, so a base work-up is needed to obtain the free aromatic amine.
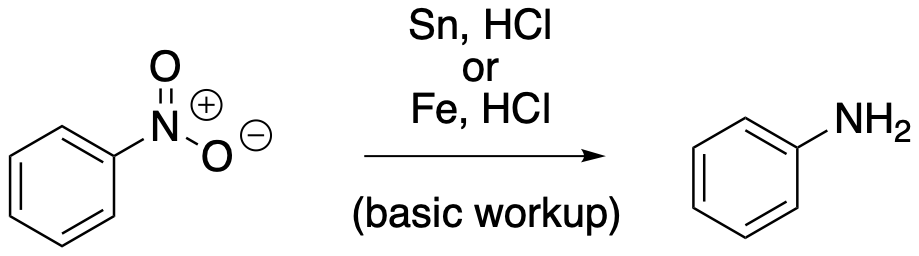
This scheme summarises the reduction of an aromatic nitro group (–NO₂) to an aniline (–NH₂) using metal/acid conditions, followed by alkali to liberate the free amine from its ammonium salt. Alternative metals are shown, but OCR requires tin and concentrated hydrochloric acid. Source
Practical Considerations for Laboratory Preparation
Aromatic amine synthesis follows standard organic preparation techniques. Reflux is used to heat the mixture without loss of volatile substances. Key practical points include:
Setting up the Reaction
Assemble a reflux apparatus with condenser and heat source.
Add nitroarene, tin metal, and concentrated HCl carefully.
Heat under reflux until the reaction mixture becomes homogeneous and reduction is complete.
After Refluxing
Once cooled:
Add alkali gradually to neutralise excess acid.
The free amine may separate as an oily layer due to limited solubility.
Extract using an organic solvent if purification is required.
Important Notes for Students
Tin/HCl reduction is the specification-required method for producing aromatic amines from nitroarenes.
The reaction must be followed by alkaline treatment to obtain the amine rather than its salt.
Aromatic amines often have strong odours and dark colours; appearance changes can indicate successful reduction.
Applications of Aromatic Amines
Aromatic amines produced by this reduction method serve as key intermediates in:
Further functional-group transformations
Production of dyes (e.g. azo dyes via diazotisation)
Pharmaceutical synthesis
Polymer and material chemistry
Understanding their preparation provides an essential foundation for more advanced synthetic pathways encountered later in the course.
FAQ
Tin is an effective electron donor in strongly acidic conditions, allowing controlled reduction of the nitro group without breaking the aromatic ring.
Concentrated hydrochloric acid maintains a high proton concentration, stabilising intermediate species during reduction and ensuring the amine is formed efficiently as its ammonium salt.
The initial reduction produces an ammonium salt rather than the free amine because the reaction occurs in acidic conditions.
Adding an alkali such as sodium hydroxide:
Neutralises excess acid
Deprotonates the ammonium ion
Releases the free aromatic amine as the final product
In aromatic amines, the lone pair on the nitrogen is partially delocalised into the aromatic ring.
This delocalisation:
Reduces availability of the lone pair to accept protons
Lowers basicity compared to aliphatic amines, where the lone pair is localised
Both reagents and products present hazards that require careful handling.
Key considerations include:
Concentrated hydrochloric acid is corrosive
Tin salts can be toxic
Aromatic amines may have strong odours and health risks
Reactions should be carried out in a fume cupboard with appropriate personal protective equipment.
Reflux allows the reaction mixture to be heated for an extended period at a constant temperature.
This ensures:
Complete reduction of the nitro group
No loss of volatile components such as acid vapours
A safer and more controlled reaction environment
Practice Questions
Nitrobenzene can be converted into phenylamine using tin and concentrated hydrochloric acid.
(a) State the role of tin in this reaction.
(b) Name the type of reaction taking place.
(2 marks)
(a) Role of tin (1 mark)
Tin acts as a reducing agent
ORTin provides electrons for the reduction
(b) Type of reaction (1 mark)
Reduction
Describe how an aromatic amine can be prepared from a nitroarene in the laboratory using reagents specified by the OCR syllabus. Your answer should include reagents, conditions, and how the free amine is obtained.
(5 marks)
Award marks as follows:
Use of tin and concentrated hydrochloric acid stated (1 mark)
Heating under reflux mentioned (1 mark)
Reduction of the nitro group to an amine stated (1 mark)
Formation of an amine (ammonium) salt under acidic conditions mentioned (1 mark)
Addition of alkali (e.g. sodium hydroxide) to liberate the free aromatic amine stated (1 mark)
Maximum 5 marks.

