OCR Specification focus:
‘Recognise and draw structures of primary and secondary amides.’
Introduction
Amides are key nitrogen-containing functional groups formed from carboxylic acids and amines; understanding primary and secondary amides is essential for structural representation, reactivity, and synthesis.
Understanding Amide Functional Groups
Amides belong to a family of compounds containing the carbonyl group (C=O) directly bonded to a nitrogen atom. They play important roles in biological molecules, synthetic polymers, and advanced organic synthesis. For OCR A-Level Chemistry, students must be able to recognise and draw both primary and secondary amides clearly and consistently.
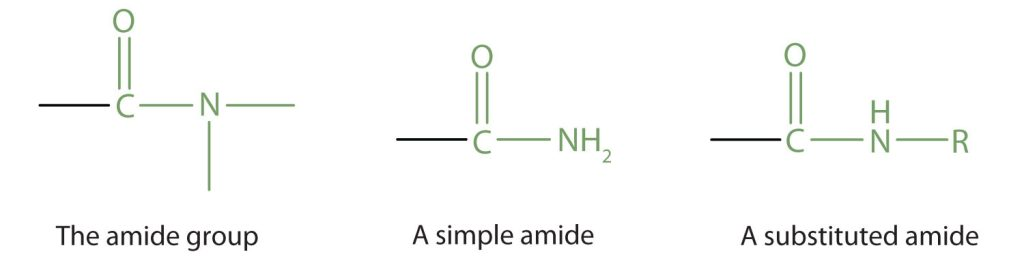
This diagram identifies the amide functional group and compares a simple amide with a substituted amide. The substituted example can represent secondary (or tertiary) amides, so it includes slightly more than the syllabus minimum. Source
The Amide Linkage
The distinctive feature of all amides is the amide linkage, which consists of a carbonyl group attached to a nitrogen atom. This arrangement significantly alters the electron distribution around the carbonyl, reducing its reactivity compared with aldehydes and ketones.
Amide: A functional group in which a carbonyl group is directly bonded to a nitrogen atom.
The presence of the nitrogen lone pair allows partial delocalisation into the carbonyl group, giving amides their characteristic resonance-stabilised structure.
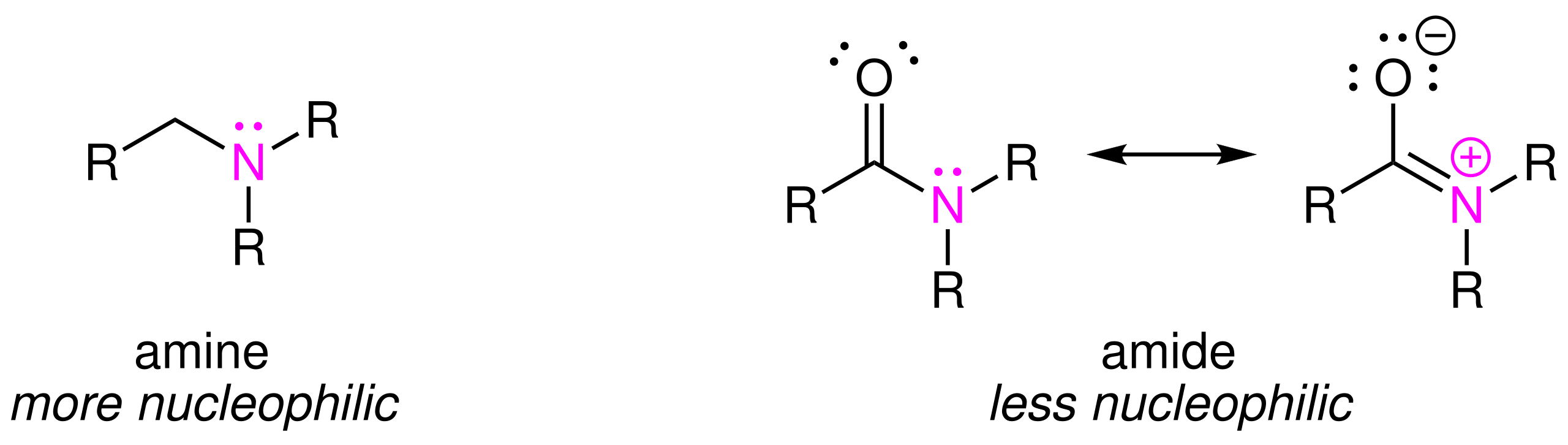
The figure contrasts an amine with two resonance structures of an amide, illustrating lone-pair delocalisation into the carbonyl group. This resonance depiction goes slightly beyond the syllabus minimum but supports the explanation in the notes. Source
Primary Amides
Primary amides contain an –CONH₂ group, meaning the nitrogen is bonded to one carbonyl carbon and two hydrogens. Students must be confident identifying this arrangement when analysing structural formulae.
Recognising Primary Amides
Primary amides are typically formed from the reaction of carboxylic acids or their derivatives with ammonia. Their structural hallmark is the NH₂ attached directly to the carbonyl carbon.
Primary Amide: An amide in which the nitrogen atom is bonded to one carbonyl group and two hydrogen atoms.
Primary amides often appear in exam questions where candidates must distinguish between different nitrogen-containing functional groups. These questions commonly assess the ability to differentiate amides from amines, nitriles, or amino acids based on structural features.
Key Features of Primary Amides
Contain the –CONH₂ functional group.
Formed via nucleophilic substitution of ammonia with acyl chlorides or acid anhydrides.
Exhibit strong hydrogen bonding due to two N–H bonds.
Display relatively high melting points and good solubility in water.
A structural representation might be written as R–CONH₂, where R can be hydrogen (giving methanamide) or an alkyl group.
Secondary Amides
Secondary amides occur when one of the hydrogens on the nitrogen is replaced by an alkyl or aryl group. This leads to important distinctions in physical properties and structural representation.
Recognising Secondary Amides
The nitrogen in a secondary amide is bonded to:
The carbonyl carbon, and
One hydrogen, and
One alkyl or aryl group.
This gives the general formula R–CONHR′, where R and R′ represent hydrocarbon groups.
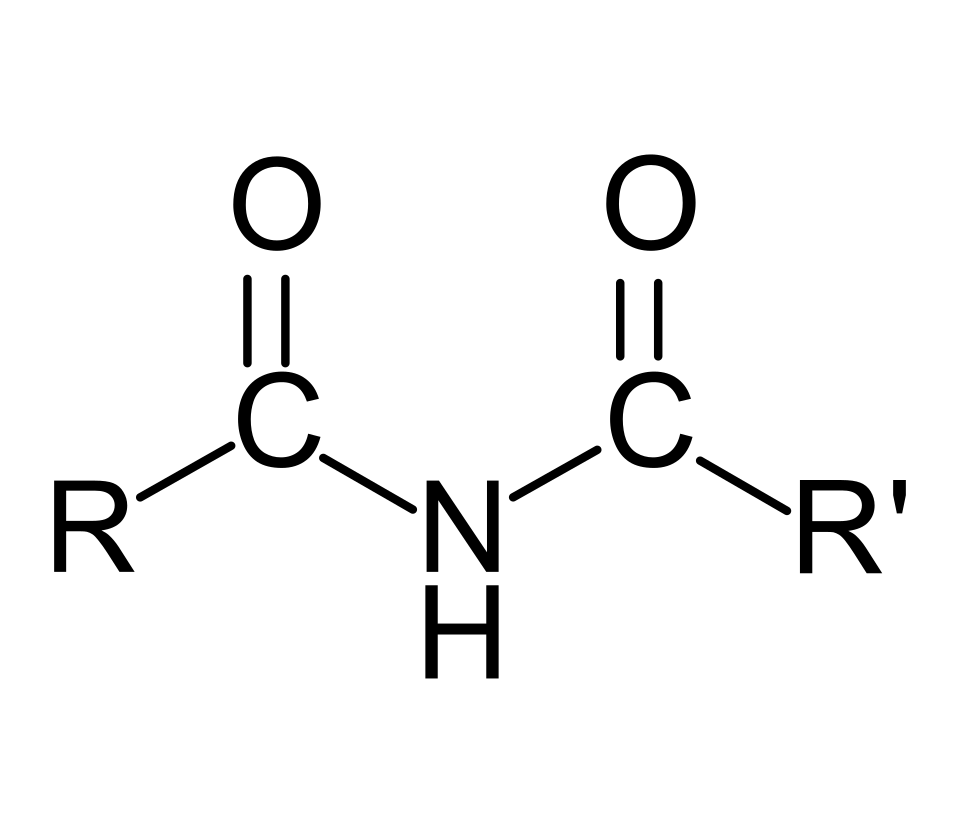
This is the general structural formula of a secondary amide, showing the carbonyl group joined to nitrogen and one N–H bond remaining. The two R groups indicate substitution on the acyl side and on the nitrogen side. Source
Secondary Amide: An amide in which the nitrogen atom is bonded to one carbonyl group, one hydrogen, and one alkyl or aryl group.
Secondary amides show slightly different infrared and boiling-point trends compared with primary amides due to the reduced number of N–H bonds.
Key Features of Secondary Amides
Contain the –CONHR functional group.
Can be formed by reacting acyl chlorides or anhydrides with primary amines.
Possess only one N–H bond, reducing hydrogen-bonding capability.
Exhibit lower melting points than primary amides of similar molar mass.
Secondary amides may appear in questions where students must distinguish them from tertiary amides, which contain no N–H bonds.
Drawing and Representing Amide Structures
Recognition and drawing of amide structures is a core requirement of the specification. Students may encounter structural, skeletal, or displayed forms.
Structural Clarity in Exam Contexts
To ensure precision:
Show the C=O group clearly.
Identify the N–H bonds if present.
Distinguish primary from secondary amides by the number of hydrogens attached to nitrogen.
Label alkyl substituents on the nitrogen where necessary.
Common Mistakes to Avoid
Misplacing the nitrogen relative to the carbonyl group.
Confusing amides with amines, which lack the carbonyl group.
Incorrectly adding substituents to the carbonyl carbon rather than the nitrogen.
Drawing tertiary amides when asked for primary or secondary structures.
Students may also be required to interpret amide functionality within larger molecules, such as peptides or synthetic polymers.
Formation of Primary and Secondary Amides
Although the specification primarily emphasises recognition and drawing, understanding how these amides are formed strengthens comprehension of their structural characteristics.
General Routes to Amides
Reaction with acyl chlorides:
Ammonia → primary amide
Primary amine → secondary amide
Reaction with acid anhydrides: Similar pathways to acyl chlorides but often milder.
Condensation reactions: Common in forming polyamides, though detailed polymerisation mechanisms belong to other subtopics.
Important Considerations in Formation
Excess ammonia promotes the formation of primary amides.
Using a primary amine introduces an alkyl group onto the nitrogen, producing a secondary amide.
Proton transfer steps are essential to stabilise intermediates in amide formation.
One sentence must follow before introducing related definitions or equations.
Acyl Chloride: A reactive derivative of a carboxylic acid in which the hydroxyl group is replaced with a chlorine atom.
This background knowledge supports accurate identification of amide types when analysing synthetic routes.
Physical and Chemical Properties Relevant to Identification
Understanding properties helps confirm the presence of primary or secondary amides when interpreting data such as infrared spectra.
Infrared Features
Primary amides: Two N–H stretching absorptions.
Secondary amides: One N–H stretching absorption.
Both types exhibit a strong C=O absorption, typically lower frequency than aldehydes due to resonance.
Hydrogen Bonding and Solubility
Primary amides form more hydrogen bonds than secondary amides.
Both types show significant intermolecular attractions, contributing to high melting points.
Amides are generally neutral, unlike amines, due to delocalisation reducing basicity.
These structural and property-based features form the foundation required for OCR A-Level Chemistry students to confidently recognise and draw primary and secondary amides as specified.
FAQ
In amides, the nitrogen lone pair is delocalised into the carbonyl group through resonance. This reduces its availability to accept protons.
In contrast, amines have a localised lone pair that is more readily donated, making them significantly more basic than amides.
Infrared spectroscopy reveals differences in N–H stretching vibrations.
Primary amides show:
Two distinct N–H stretching peaks
Secondary amides show:
One N–H stretching peak
Both show a strong C=O absorption, but the number of N–H peaks allows clear differentiation.
Primary amides form more extensive hydrogen bonding networks because they contain two N–H bonds.
This increases intermolecular attraction, requiring more energy to separate molecules and resulting in higher melting points compared with secondary amides of similar size.
Replacing a hydrogen on the nitrogen with an alkyl group slightly reduces overall polarity.
This is because:
Fewer N–H bonds are available for hydrogen bonding
Alkyl groups are non-polar and reduce intermolecular attraction
As a result, secondary amides are often less soluble in water than primary amides.
The amide linkage is chemically stable due to resonance, making it resistant to spontaneous breakdown.
This stability allows long chains of amino acids to remain intact, forming peptide bonds that maintain protein structure while still permitting controlled reactions under biological conditions.
Practice Questions
The structure below represents an amide.
CH3CONHCH3
(a) Identify the type of amide shown.
(b) State one structural feature that distinguishes this amide from a primary amide.
(2 marks)
(a)
Secondary amide (1 mark)
(b)
Nitrogen bonded to one alkyl group and one hydrogen (1 mark)
OROnly one N–H bond present (1 mark)
Amides are commonly encountered in organic synthesis.
(a) Draw the general structural formula of a primary amide using R notation.
(b) Draw the general structural formula of a secondary amide using R notation.
(c) Explain, using structure, how a primary amide differs from a secondary amide.
(d) State one reason why primary amides can form more hydrogen bonds than secondary amides.
(5 marks)
(a)
Correct general formula shown as RCONH2 (1 mark)
(b)
Correct general formula shown as RCONHR or RCONHR′ (1 mark)
(c)
Primary amide has two hydrogens attached to nitrogen (1 mark)
Secondary amide has one hydrogen and one alkyl/aryl group attached to nitrogen (1 mark)
(d)
Primary amides have two N–H bonds available for hydrogen bonding (1 mark)

