OCR Specification focus:
‘Use RCH(NH2)COOH; carboxyl group forms esters and reacts with alkalis; amine group reacts with acids.’
Introduction
α-Amino acids contain both amine and carboxyl groups attached to the same carbon atom, giving them distinctive chemical behaviour essential for biological function and synthetic transformations.
Structure of α-Amino Acids
α-Amino acids follow the general formula RCH(NH₂)COOH, where the α-carbon is bonded to four different groups: an amine group, a carboxyl group, a hydrogen atom, and a variable R group.
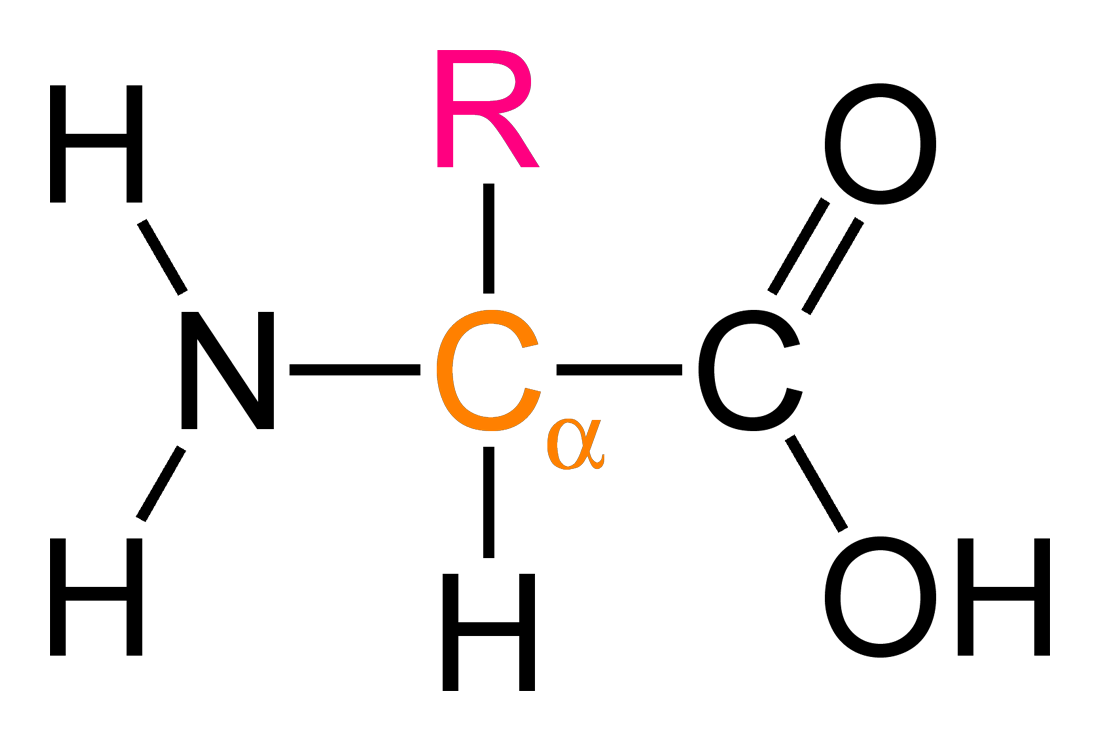
This diagram shows the general structure of an α-amino acid, highlighting the α-carbon bonded to an amine group, a carboxyl group, a hydrogen atom, and a variable R group. The R group differs between amino acids and determines their individual properties. Source
Functional Groups Present
Amine group (–NH₂)
Carboxyl group (–COOH)
Side chain (R group), which determines the identity and properties of each amino acid
Key Terminology
Before continuing, it is essential to define the fundamental terms associated with α-amino acids.
Functional group: A specific group of atoms within a molecule responsible for its characteristic chemical reactions.
The combination of an amine group and carboxyl group in the same molecule creates both acidic and basic behaviour, giving α-amino acids their characteristic amphoteric nature.
The α-Carbon
The α-carbon is positioned adjacent to the carboxyl group. For standard α-amino acids (except glycine, where R = H), this carbon is bonded to four different substituents, allowing chirality, although chirality is covered elsewhere in the specification.
Behaviour of the Carboxyl Group (–COOH)
The carboxyl group of an α-amino acid behaves similarly to all carboxylic acids: it is acidic, donates protons, and undergoes esterification and salt formation.
Reaction with Alcohols: Esterification
The specification states that the carboxyl group forms esters. In the presence of an alcohol and an acid catalyst (commonly concentrated H₂SO₄), esterification occurs.
Produces esters and water
The amine group must usually be protonated beforehand to prevent side reactions
Esterification: A condensation reaction between a carboxylic acid and an alcohol to form an ester and water.
This transformation is important because amino acid esters are used in peptide synthesis, protecting the carboxyl group during multi-step procedures.
Reaction with Alkalis
The specification emphasises that the carboxyl group reacts with alkalis. This reaction forms carboxylate salts, which are ionic and soluble in water.
Reaction with NaOH yields RCH(NH₂)COO⁻Na⁺
This process is typical of all carboxylic acids
This salt formation is useful for purification and increasing solubility of amino acids in aqueous systems.
Behaviour of the Amine Group (–NH₂)
Amino groups are basic because their nitrogen atom has a lone pair, enabling proton acceptance. This behaviour is central to how α-amino acids interact with acids.
Reaction with Acids: Salt Formation
The specification states that the amine group reacts with acids. When treated with dilute acids such as HCl, the amine group is protonated to form an ammonium salt.
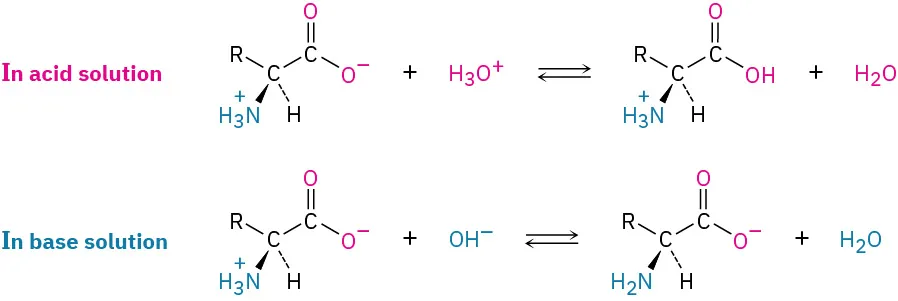
The diagram illustrates how an amino acid’s functional groups respond to acidic and basic conditions. In acid solution, the amine group is protonated, while in base solution the carboxyl group loses a proton, demonstrating amphoteric behaviour. Source
Produces RCH(NH₃⁺)COOH Cl⁻
Reaction increases solubility in aqueous acidic environments
Protonation: The addition of a proton (H⁺) to an atom, molecule, or ion.
Protonating the amine group is important in peptide synthesis and in adjusting solubility for separation techniques.
Zwitterion Formation
α-Amino acids contain both acidic and basic groups, enabling them to form zwitterions, molecules with both positive and negative charges but overall electrical neutrality. This behaviour is related to, but not explicitly stated in, the specification; however, understanding functional group reactivity naturally leads to recognising zwitterionic structure.
Carboxyl group donates a proton → COO⁻
Amine group accepts a proton → NH₃⁺
Internal transfer produces a dipolar ion
Zwitterion: A molecule containing both positive and negative charges but overall electrically neutral.
Amino acids in zwitterionic form display high melting points and good solubility in water due to strong ionic interactions.
Acid–Base Properties of α-Amino Acids
The dual presence of acidic and basic groups gives rise to amphoteric behaviour.
Amphoteric Nature
Act as acids by donating a proton from the carboxyl group
Act as bases by accepting a proton on the amine group
This dual reactivity underpins why amino acids interact differently under acidic, neutral, and basic conditions.
Reaction Summary
Carboxyl group + alkali → carboxylate salt
Carboxyl group + alcohol + acid catalyst → ester
Amine group + acid → ammonium salt
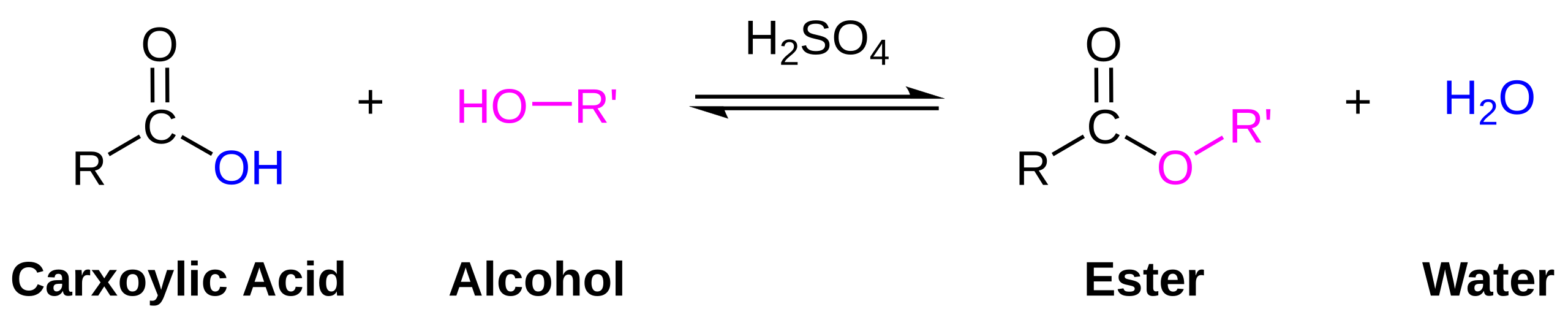
This diagram shows the overall esterification reaction between a carboxylic acid and an alcohol, producing an ester and water. Although shown generically, the same transformation applies to the carboxyl group of amino acids under acidic conditions. Source
These reactions form the foundation of amino acid chemistry and link directly to synthetic applications.
Importance of R Group Variation
Although the specification focuses on core functional groups, it is important to recognise that R groups influence reactivity and physical properties.
Influence on Solubility and Reactivity
Polar R groups increase solubility
Non-polar R groups decrease solubility
Acidic or basic R groups introduce additional reactive sites
This variation plays a major role in biochemical behaviour, though OCR A-Level primarily emphasises recognition of core reactions involving the amine and carboxyl groups.
Summary of Specification Links
The OCR specification for this subsubtopic requires clear understanding of the following:
Structure of α-amino acids using RCH(NH₂)COOH
Carboxyl group reactions: ester formation and reaction with alkalis
Amine group reactions: salt formation with acids
These reactions define the chemical properties of α-amino acids and form the foundation for their behaviour in organic and biological systems.
FAQ
α-Amino acids exist mainly as zwitterions in the solid state, rather than as neutral molecules.
This means they contain both positive and negative charges within the same molecule, leading to strong ionic attractions between neighbouring particles.
These electrostatic forces require significantly more energy to overcome than the intermolecular forces found in non-ionic organic compounds, resulting in higher melting points.
The amine group is basic and can react with acids used in esterification.
If unprotected, it may:
Form an ammonium salt instead of allowing ester formation
Interfere with the reaction mechanism
Protonating or protecting the amine group ensures the reaction occurs specifically at the carboxyl group, improving yield and selectivity.
α-Amino acids are generally highly soluble in water due to their zwitterionic nature.
The charged groups interact strongly with polar water molecules through ion–dipole attractions.
Solubility can vary depending on the R group, with polar side chains increasing solubility and non-polar side chains reducing it.
Because both functional groups are present in the same molecule, they influence each other’s behaviour.
Internal proton transfer can occur, forming zwitterions rather than free acids or bases.
This means reactions and properties such as boiling point, solubility, and acid–base behaviour differ from simple amines or carboxylic acids.
The reacting functional group depends on the conditions used.
For example:
Acidic conditions favour reactions involving the amine group
Alkaline conditions favour reactions of the carboxyl group
Controlled acidic conditions allow esterification of the carboxyl group
Careful choice of conditions allows selective reactions despite both groups being present.
Practice Questions
An α-amino acid has the general formula RCH(NH₂)COOH.
Explain why an α-amino acid can react with both acids and alkalis.
(2 marks)
Award marks as follows:
Identifies the presence of both an amine group and a carboxyl group (1 mark)
Explains that the amine group can accept a proton (basic) and the carboxyl group can donate a proton (acidic), or equivalent explanation of amphoteric behaviour (1 mark)
Describe and explain the reactions of an α-amino acid with:
a) a dilute acid
b) an alkali
c) an alcohol in the presence of an acid catalyst
In your answer, refer to the functional groups involved and the type of products formed.
(5 marks)
Marks should be awarded for clear, chemically accurate statements.
a) Reaction with a dilute acid (2 marks)
States that the amine group is protonated / reacts with the acid (1 mark)
Identifies formation of an ammonium salt or shows NH₂ becoming NH₃⁺ (1 mark)
b) Reaction with an alkali (2 marks)
States that the carboxyl group reacts with the alkali / donates a proton (1 mark)
Identifies formation of a carboxylate salt, e.g. COO⁻ with a metal ion (1 mark)
c) Reaction with an alcohol and acid catalyst (1 mark)
States that the carboxyl group undergoes esterification to form an ester (1 mark)
Maximum total: 5 marks

