Understanding atomic structure and how electrons are arranged in atoms is crucial to mastering chemistry and biology concepts, especially bonding and periodic behavior.
Atomic Structure: A Quick Recap
Atoms are the fundamental units of matter and are composed of three types of subatomic particles: protons, neutrons, and electrons. These particles differ in mass, charge, and location within the atom.
Protons are found in the nucleus and have a positive charge (+1). Each element has a unique number of protons, known as its atomic number, which determines the element's identity. Protons have a mass close to 1 atomic mass unit (amu).
Neutrons, also located in the nucleus, have no charge (neutral) and a similar mass to protons (~1 amu). Neutrons contribute to the atom’s mass number, which is the sum of protons and neutrons.
Electrons orbit the nucleus in regions called orbitals and have a negative charge (-1). Their mass is nearly negligible compared to protons and neutrons (~0 amu). In a neutral atom, the number of electrons is equal to the number of protons.
Electrons are not randomly distributed but occupy defined energy levels around the nucleus. These levels are structured and help explain the chemical behavior of elements.
Dalton’s Atomic Theory
Developed in the early 1800s, John Dalton’s Atomic Theory laid the groundwork for modern atomic science. Although later discoveries have refined the theory, its core concepts are still essential:
All matter is composed of small indivisible particles called atoms.
All atoms of a given element are identical in mass and properties.
Compounds are combinations of atoms in fixed, whole-number ratios.
Chemical reactions involve rearrangements of atoms but do not change the atoms themselves.

The structure of an atom! We can see the protons and neutrons in the nucleus, with electrons orbiting it.
Dalton's ideas introduced the concept that atoms are conserved in chemical reactions, forming the basis of the law of conservation of mass.
Coulomb’s Law and Atomic Interactions
To understand how atomic particles interact, particularly the attraction between negatively charged electrons and positively charged protons, we use Coulomb’s Law. It quantifies the electric force between two charged particles:
Coulomb’s Law:
Fe = k (q1 q2) / r²

Image Courtesy of APlusPhysics
Where:
Fe is the electric force between the charges,
k is Coulomb’s constant,
q1 and q2 are the magnitudes of the charges,
r is the distance between the centers (nuclei) of the two particles.
Key implications of Coulomb’s Law:
A larger charge results in a stronger force.
A smaller distance between particles results in a stronger attraction.
This explains why inner electrons are held more tightly than valence electrons, which are farther from the nucleus and experience less electrostatic attraction.
The Bohr Model of the Atom

Image Courtesy of Wikimedia_Bohr_model.png)
Niels Bohr developed a model where electrons travel in fixed orbits around the nucleus. These orbits correspond to specific energy levels, and electrons in higher orbits possess more energy. According to Bohr:
Electrons in atoms exist only in certain allowed energy levels.
Electrons closer to the nucleus have lower energy.
Electrons can move between levels by absorbing or releasing energy in fixed quantities, called quanta.
Bohr's model helped explain the line spectra of elements and introduced the idea of quantized energy levels.
In a Bohr diagram, each ring represents an energy level or electron shell:
For example, sodium (Na) has an atomic number of 11, so it has 11 electrons:
2 in the first shell,
8 in the second shell,
1 in the third shell (valence shell).
This one valence electron explains sodium’s reactivity, as atoms tend to gain or lose electrons to achieve a full outer shell.
Electron Configuration: The Basics
Electron configuration describes how electrons are arranged within an atom’s orbitals. This arrangement follows specific principles and is key to understanding chemical bonding, reactivity, and periodic trends.
Electrons fill:
Principal energy levels (n = 1, 2, 3…), increasing in distance from the nucleus.
Sublevels (s, p, d, f) within each energy level, which have different shapes and capacities:
s holds up to 2 electrons,
p holds up to 6,
d holds up to 10,
f holds up to 14.
Core vs. Valence Electrons
Core electrons are found in inner, filled energy levels and are not involved in chemical bonding.
Valence electrons occupy the outermost s and p orbitals and are responsible for the atom’s chemical behavior.
Example:
Sulfur (S) has the electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁴
Core electrons: 1s² 2s² 2p⁶ (10 electrons)
Valence electrons: 3s² 3p⁴ (6 electrons)
Valence electrons determine properties like reactivity, bonding, and ion formation.
The Periodic Table and Subshells

The periodic table is designed to reflect the order in which electrons fill orbitals:
s-block: Groups 1 and 2
p-block: Groups 13 to 18
d-block: Transition metals (Groups 3 to 12)
f-block: Lanthanides and actinides
This structure helps when writing electron configurations and predicting element properties.
Rules for Writing Electron Configurations

1. Aufbau Principle
Electrons occupy the lowest energy orbitals first, moving to higher energy levels only after lower ones are full. The typical order is:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p
This sequence follows increasing energy, not just numerical order.
2. Pauli Exclusion Principle
No two electrons in the same atom can have identical quantum numbers. Therefore, an orbital can hold a maximum of two electrons, and they must have opposite spins (usually represented as ↑ and ↓).
3. Hund’s Rule
When electrons occupy orbitals of the same sublevel (like three p orbitals), they fill each orbital singly first, all with the same spin. Only after all orbitals in the sublevel are half-filled do electrons pair up.
This rule minimizes repulsion between electrons and stabilizes the atom.
Step-by-Step: Writing Electron Configurations
Example: Boron (B), Atomic Number = 5
Total electrons = 5
Fill in order:
1s²
2s²
2p¹
Final configuration: 1s² 2s² 2p¹
The superscripts add up to 5, matching the atomic number. Each sublevel indicates the number and location of electrons.
Using the Noble Gas Shortcut
For larger atoms, the noble gas configuration simplifies the process:
Identify the closest noble gas before the element.
Use its symbol in brackets.
Add remaining configuration from there.
Example: Boron
Nearest noble gas: Helium (He)
Shortcut: [He] 2s² 2p¹
Example: Iron (Fe), Atomic Number = 26
Full configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
Noble gas shortcut: [Ar] 4s² 3d⁶

Notice that although 3d comes after 4s, it has higher energy, so it’s filled afterward.

Orbital Diagrams
Orbital diagrams are visual representations of how electrons fill orbitals. Each orbital is shown as a box, and electrons are arrows:
Image Courtesy of Chegg

↑ or ↓ indicates electron spin
Boxes are labeled with subshells: 1s, 2s, 2p, etc.
Example: Carbon (C), Atomic Number = 6
1s: ↑↓
2s: ↑↓
2p: ↑ ↑
In the 2p sublevel, two electrons occupy separate orbitals with parallel spins (Hund’s Rule).
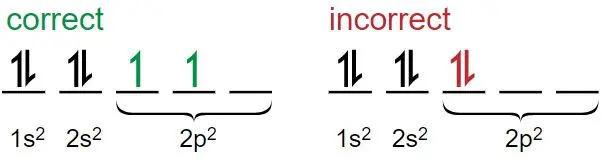
Image Courtesy of Chemistry 301
Valence Electrons and Reactivity

The number of valence electrons influences:
Bond formation (ionic or covalent),
Ion charges,
Chemical reactivity.
To find valence electrons:
Identify the highest energy level (largest n value).
Count the electrons in s and p orbitals of that level.
Example: Arsenic (As)
Electron configuration: [Ar] 4s² 3d¹⁰ 4p³
n = 4 is the highest energy level.
Valence electrons = 4s² + 4p³ = 5 valence electrons
This explains arsenic’s tendency to form three or five bonds in compounds.
Essential Vocabulary
Atomic Theory: All matter is made of atoms, which are indivisible in chemical reactions.
Bohr Model: Electrons occupy fixed orbits around the nucleus with quantized energy.
Electron Configuration: The arrangement of electrons in orbitals of an atom.
Aufbau Principle: Electrons fill orbitals in order of increasing energy.
Pauli Exclusion Principle: No two electrons in an atom can have identical quantum states.
Hund’s Rule: Electrons fill empty orbitals before pairing up in a sublevel.
Valence Electrons: Outer shell electrons that determine chemical behavior.
Core Electrons: Inner electrons not involved in bonding.
Orbitals: Regions around the nucleus where electrons are likely to be found.
Subshells (s, p, d, f): Divisions of electron shells with specific capacities.
Coulomb’s Law: Describes the electric force between charged particles.
Noble Gas Shortcut: A method for writing shortened electron configurations.
FAQ
Although the 3d orbital is part of the third energy level and the 4s is part of the fourth, the 4s orbital is actually lower in energy than the 3d orbital when orbitals are empty or partially filled. This is because the 4s orbital has a slightly higher penetration toward the nucleus and experiences less shielding from inner electrons compared to the 3d orbital. As a result, electrons fill the 4s orbital first according to the Aufbau Principle. However, once electrons start populating the 3d orbitals, the energy levels can shift, and 3d may become lower in energy than 4s.
The 4s orbital has a lower energy due to less electron shielding.
Electron-electron interactions in multi-electron atoms affect orbital energy order.
Once 3d begins filling, the 4s orbital often becomes higher in energy and is lost first during ionization.
Transition metals often lose electrons from the 4s orbital before the 3d orbitals when forming ions, even though 4s fills first. This happens because once the 3d orbitals start filling, they become lower in energy than the 4s orbital due to electron repulsion and changes in effective nuclear charge. As a result, when transition metals are ionized:
The 4s electrons are removed first.
This leads to unusual oxidation states common in transition metals.
For example, iron (Fe) has a ground-state configuration of [Ar] 4s² 3d⁶, but forms Fe²⁺ as [Ar] 3d⁶ and Fe³⁺ as [Ar] 3d⁵.
This explains the versatility and multiple oxidation states of transition metals.
Certain elements, particularly transition metals like chromium (Cr) and copper (Cu), exhibit irregular electron configurations that deviate from the expected Aufbau order to increase stability. This occurs because half-filled and fully-filled subshells offer extra stability due to symmetrical electron distribution and minimized repulsion.
Chromium is expected to be [Ar] 4s² 3d⁴ but is actually [Ar] 4s¹ 3d⁵.
Copper is expected to be [Ar] 4s² 3d⁹ but is actually [Ar] 4s¹ 3d¹⁰.
These configurations result in more stable, lower-energy arrangements.
The slight energy trade-off in promoting an electron from 4s to 3d is compensated by the resulting stability of half-filled or fully filled d orbitals.
Effective nuclear charge (Zeff) refers to the net positive charge felt by an electron from the nucleus, considering the shielding effect of other electrons. Zeff increases across a period as more protons are added to the nucleus without significant increases in shielding. This affects electron configuration in the following ways:
Outer electrons are drawn closer to the nucleus, decreasing atomic radius.
Electrons are held more tightly, raising ionization energy.
Filling of subshells occurs in a way that balances energy minimization and electron repulsion.
Higher Zeff also explains the ordering of orbital energies and why orbitals like 2s are lower in energy than 2p, despite being in the same energy level.
When atoms gain or lose electrons to form ions, their electron configurations adjust to reflect the change in electron number, usually in a way that achieves a full outer shell (octet rule). The specific changes depend on whether the ion is a cation (positive) or anion (negative).
Cations: Electrons are removed from the outermost shell first (usually s or p orbitals).
Example: Mg (1s² 2s² 2p⁶ 3s²) becomes Mg²⁺ (1s² 2s² 2p⁶).
Anions: Electrons are added to the lowest available empty orbitals.
Example: Cl (1s² 2s² 2p⁶ 3s² 3p⁵) becomes Cl⁻ (1s² 2s² 2p⁶ 3s² 3p⁶).
Main-group elements tend to form ions that mimic noble gas configurations, achieving maximum stability with filled valence shells. Transition metals often deviate due to involvement of d orbitals.
Practice Questions
Explain how the electron configuration of an atom influences its chemical reactivity. Use an example to support your answer.
The electron configuration determines how many valence electrons an atom has, which directly affects its chemical reactivity. Atoms tend to gain, lose, or share electrons to achieve a full outer shell, typically resembling the nearest noble gas configuration. For example, sodium (Na) has one valence electron in the 3s orbital (1s² 2s² 2p⁶ 3s¹). Because it only needs to lose one electron to achieve a stable configuration like neon (Ne), it readily loses this electron to form a Na⁺ ion. This high tendency to lose one electron makes sodium highly reactive, especially with halogens like chlorine.
Describe the principles that govern the order in which electrons fill atomic orbitals. How do these principles determine the electron configuration of oxygen?
Electrons fill atomic orbitals based on the Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule. The Aufbau Principle states electrons occupy the lowest available energy levels first. The Pauli Exclusion Principle ensures no two electrons in an atom have the same quantum numbers, so each orbital holds a maximum of two electrons with opposite spins. Hund’s Rule states that electrons fill degenerate orbitals singly before pairing. Oxygen has 8 electrons, and its configuration is 1s² 2s² 2p⁴. The 2p orbitals will have two paired and two unpaired electrons due to these rules, influencing its bonding and paramagnetic properties.

