IB Syllabus focus:
‘Most urban pollutants derive directly or indirectly from burning fossil fuels; tropospheric ozone forms secondarily from precursors.’
Urban air pollution is strongly linked to human reliance on fossil fuels. Combustion processes generate pollutants that dominate urban air quality problems, impacting health, ecosystems, and climate stability.
Fossil Fuels as a Primary Source of Urban Pollutants
Fossil fuels—coal, oil, and natural gas—are the foundation of modern energy systems. When burned in vehicles, industries, and power plants, they emit a wide range of primary pollutants. These include carbon monoxide (CO), sulphur dioxide (SO₂), nitrogen oxides (NOₓ), and particulate matter (PM10, PM2.5).
Why fossil fuels dominate
Global reliance on energy: Cities depend on fossil fuels for transport, electricity, heating, and manufacturing.
High energy density: Fossil fuels provide concentrated energy, meaning more emissions per unit of activity.
Urban concentration: Dense populations and industries lead to concentrated pollutant emissions.
Primary Pollutants: Pollutants directly emitted into the atmosphere from a source, such as CO, SO₂, NOₓ, and particulates from fossil-fuel burning.
Secondary Pollutants and Tropospheric Ozone
Not all pollutants are directly emitted. Some form through chemical reactions in the atmosphere. Tropospheric ozone (O₃) is the most significant secondary pollutant linked to fossil fuel combustion.
Formation process:
NOₓ and volatile organic compounds (VOCs) react under sunlight.
These reactions produce ozone in the lower atmosphere.
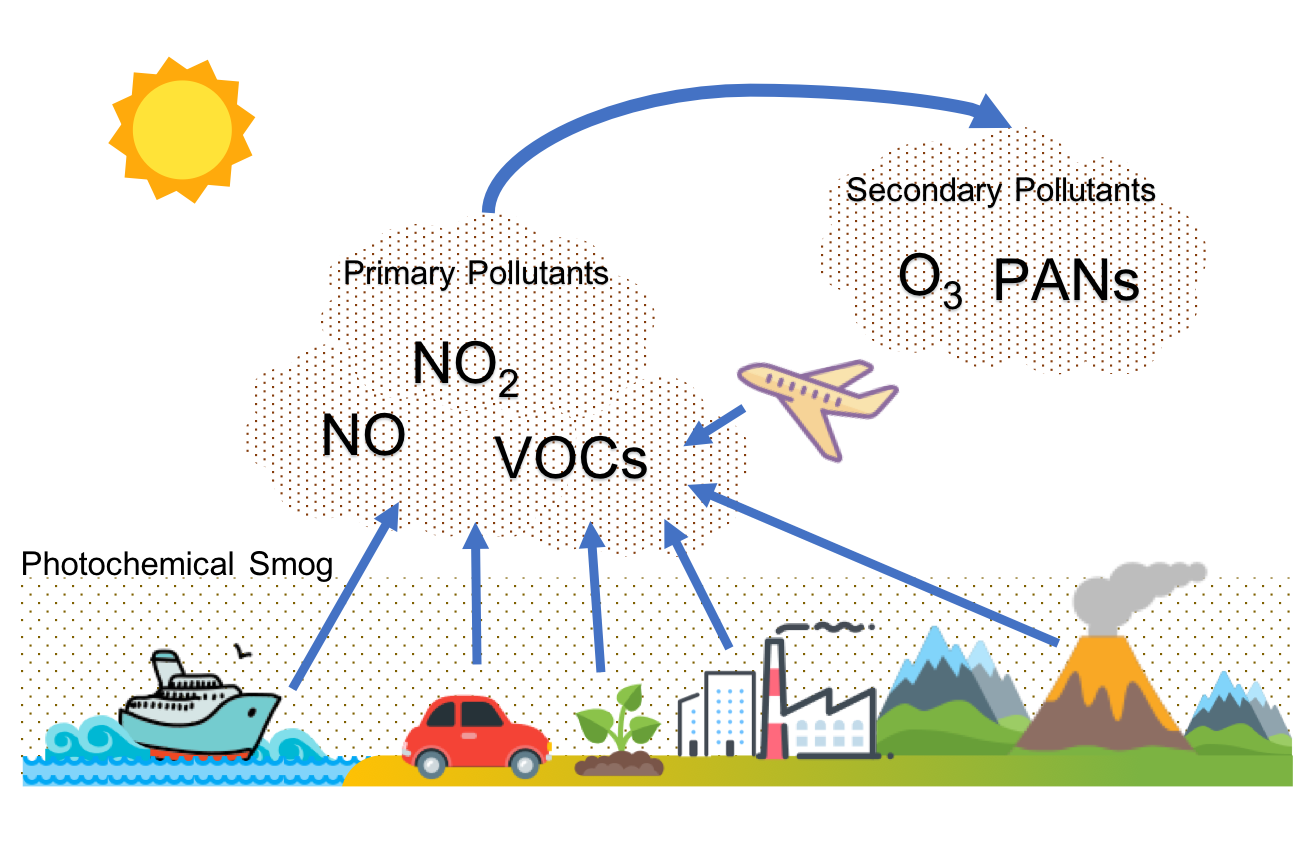
Diagram showing nitrogen oxides and volatile organic compounds from vehicles and industry reacting in sunlight to produce ground-level ozone and photochemical smog. Labels make the precursor–product pathway explicit, aligning with the IB requirement that urban O₃ forms secondarily. Minor iconography (e.g., sun, car, factory) is included only to clarify sources and sunlight’s role. Source.
Urban smog: Tropospheric ozone is a key component of photochemical smog, often seen in major cities.
Secondary Pollutants: Pollutants not emitted directly but formed in the atmosphere through reactions between primary pollutants and other atmospheric components.
Dominant Pollutants from Fossil Fuel Combustion
Carbon Monoxide (CO)
Produced by incomplete combustion, especially from vehicles. It binds with haemoglobin, reducing oxygen transport in blood.
Sulphur Dioxide (SO₂)
Emitted mainly from coal burning in power stations. It contributes to acid deposition and respiratory irritation.
Nitrogen Oxides (NOₓ)
Generated by high-temperature combustion in engines and industry. They play a major role in ozone formation and acid rain.
Particulate Matter (PM10 and PM2.5)
Tiny particles from combustion, industrial dust, and road emissions. They penetrate deep into lungs, causing cardiovascular and respiratory diseases.
PM10: Particles with a diameter of 10 micrometres or less.
PM2.5: Finer particles, 2.5 micrometres or less, more harmful to health.
Fossil Fuel Combustion and Global Trends
Energy and transport
Vehicles: Cars, buses, and lorries account for large shares of CO, NOₓ, and particulates.
Power generation: Coal remains a major source of SO₂ and CO₂.
Industry: Factories and refineries contribute a mix of pollutants.
Rising urban demand
Rapid urbanisation leads to increased energy use.
Developing nations often rely heavily on coal and oil.
Cities in Asia and Africa are experiencing sharp increases in air pollution from fossil fuels.
The Role of Tropospheric Ozone in Air Quality
Tropospheric ozone exemplifies how fossil fuel use indirectly worsens air quality. Unlike stratospheric ozone (protective), ground-level ozone is harmful.
Impacts on health: Irritates eyes, lungs, and increases asthma cases.
Impacts on plants: Damages leaf tissues, reducing crop yields and biodiversity.
Climate effects: Acts as a greenhouse gas, contributing to warming.
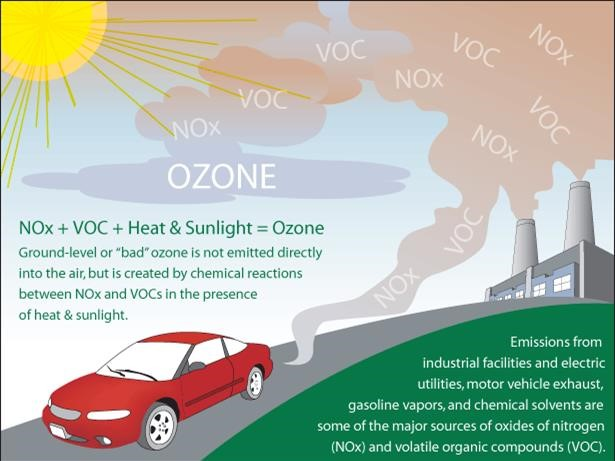
Graphic depicting NOₓ and VOC emissions from vehicles and industrial sources reacting in sunlight to create ground-level ozone, a major component of urban photochemical smog. This visual focuses on the secondary formation pathway highlighted in the syllabus. The figure includes simple icons and arrows to emphasise precursor flows. Source.
Why Fossil Fuels Are Hard to Replace
Despite their environmental costs, fossil fuels dominate because:
Infrastructure is built around them.
Alternatives such as renewables require investment.
Political and economic pressures maintain dependency.
Energy transition challenges
Urban transport still heavily relies on petroleum fuels.
Electricity grids in many countries depend on coal.
Industrial processes, such as steel and cement production, are energy-intensive.
Interconnected Systems of Pollution
Urban air pollution is not isolated. Fossil fuel combustion contributes simultaneously to:
Air quality degradation: Smog, ozone, particulates.
Acid deposition: SO₂ and NOₓ reacting with water vapour.
Climate change: CO₂ emissions driving global warming.
This interconnectedness highlights why fossil fuel combustion is identified as the dominant cause of urban pollution.
Key Takeaways for IB ESS
Fossil fuels are the main source of urban air pollutants.
Primary pollutants: CO, SO₂, NOₓ, PM10, PM2.5.
Secondary pollutants: Especially tropospheric ozone formed from precursors.
Urbanisation intensifies reliance on fossil fuels, worsening pollution.
Links to health, ecosystem damage, and global climate impacts make fossil fuels central to urban pollution studies.
FAQ
Urban areas have higher population densities, more vehicles, and intensive industrial activity, all of which rely heavily on fossil fuels.
Tall buildings can trap pollutants between them, creating "street canyons" where emissions accumulate. Limited vegetation in cities also reduces the natural absorption and dispersion of pollutants.
While ozone is a key component, fossil fuel combustion also releases fine particulates, nitrogen oxides, and hydrocarbons.
Together, these interact under sunlight to produce a brown haze, reducing visibility, irritating the respiratory system, and creating harmful secondary compounds such as peroxyacetyl nitrates (PANs).
Unlike primary pollutants, ozone is highly reactive. It penetrates deeply into lungs and damages cell membranes, causing oxidative stress.
It also persists over wide areas, travelling hundreds of kilometres from its source, making its effects broader than pollutants that stay close to urban centres.
Temperature inversions trap pollutants near the ground, preventing dispersion.
Strong sunlight accelerates photochemical reactions that generate ozone.
Low wind speeds reduce pollutant dispersal, leading to accumulation.
These conditions often occur in valleys or basins, where cities such as Los Angeles experience chronic smog.
Countries with strict fuel standards require cleaner fuels with reduced sulphur and lead content. This lowers emissions of SO₂ and toxic particulates.
In contrast, regions with weaker regulations often use high-sulphur coal or low-grade petrol and diesel, producing higher levels of pollutants and worsening ozone formation.
Practice Questions
Question 1 (2 marks)
Identify two primary pollutants produced by the combustion of fossil fuels in urban areas.
Mark scheme:
1 mark for each correct primary pollutant, up to 2 marks.
Acceptable answers: carbon monoxide (CO), sulphur dioxide (SO₂), nitrogen oxides (NOₓ), particulate matter (PM10 or PM2.5).
Do not award marks for secondary pollutants (e.g. ozone).
Question 2 (5 marks)
Explain how fossil fuel combustion leads to the formation of tropospheric ozone, and discuss two negative impacts of this secondary pollutant on human health and the environment.
Mark scheme:
Up to 2 marks for explaining the process of ozone formation:
(1 mark) Fossil fuel combustion produces NOₓ and VOCs.
(1 mark) These react in the presence of sunlight to form tropospheric ozone.
Up to 3 marks for impacts (1 mark each, maximum 2 marks for human health, maximum 2 marks for environment):
Human health: respiratory irritation, aggravation of asthma, reduced lung function, eye irritation.
Environment: crop yield reduction, damage to plant tissues, biodiversity loss, contribution to climate change as a greenhouse gas.
Maximum 5 marks in total.

