IB Syllabus focus:
‘NOx and SO2 react with oxygen and water in air to form nitric and sulfuric acids, leading to acid deposition.’
Acid rain forms when atmospheric pollutants undergo chemical reactions producing acidic compounds. This process highlights the link between human activity, fossil fuel combustion, and wider environmental impacts across ecosystems and society.
The Chemistry of Acid Rain Formation
Key Pollutants
The main contributors to acid rain formation are nitrogen oxides (NOx) and sulphur dioxide (SO2). These gases are released predominantly through fossil fuel combustion, particularly from power stations, industrial activities, and vehicles.
Nitrogen oxides (NOx): A group of gases including nitrogen monoxide (NO) and nitrogen dioxide (NO2) formed mainly during high-temperature combustion.
Sulphur dioxide (SO2): A colourless, pungent gas produced by the burning of coal and oil containing sulphur impurities and by volcanic eruptions.
These gases are precursors to acidic compounds that are deposited in rainfall or as dry particles.
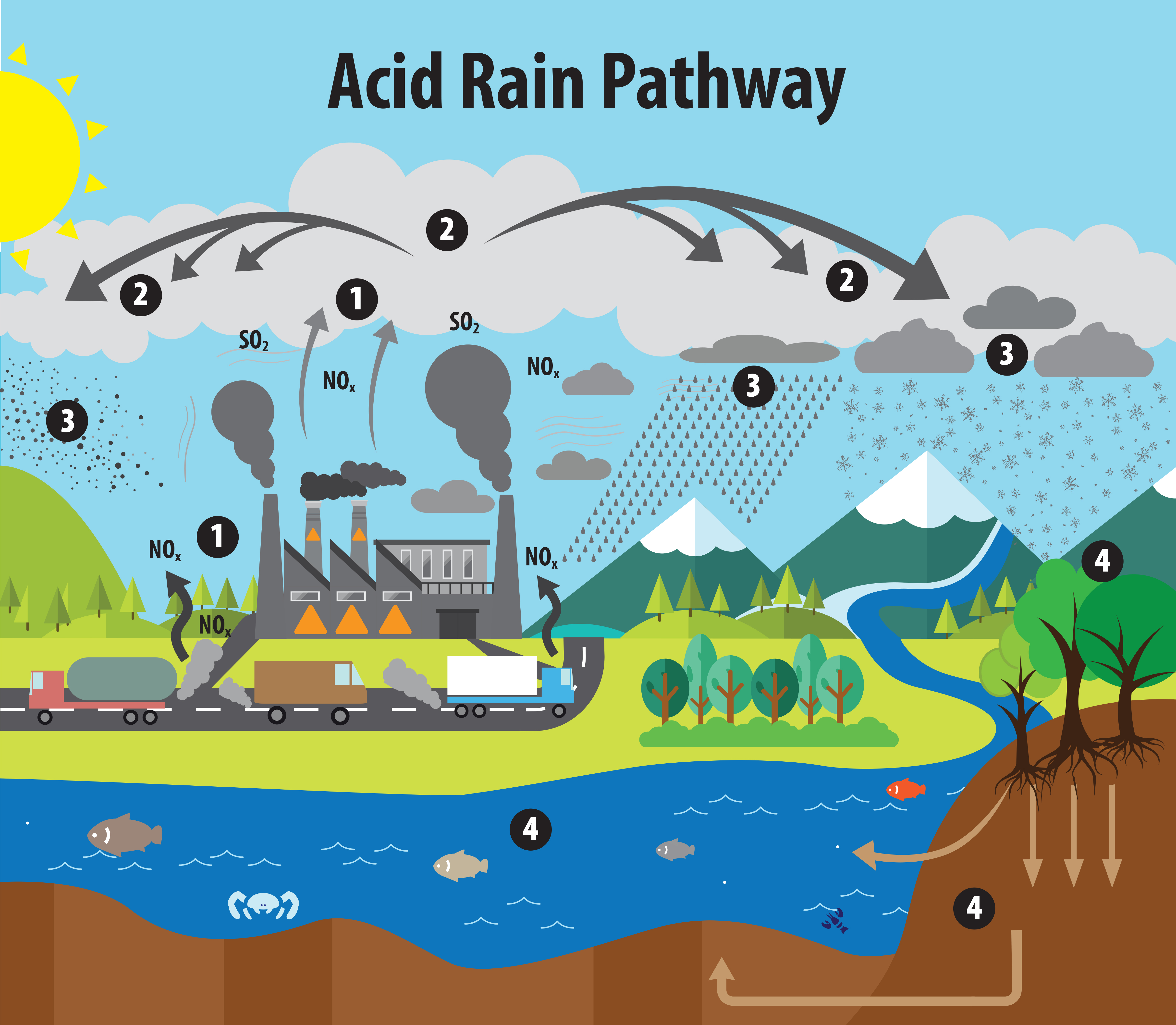
Diagram of the acid-rain pathway: SO₂ and NOx from combustion rise, react with oxygen and water to form H₂SO₄ and HNO₃, and are deposited as wet or dry deposition. Arrows show transport and transformation, matching the chemistry described for acid rain formation. The figure includes deposition effects icons beyond the formation step, which exceed the narrow subsubtopic but aid context. Source.
Oxidation Reactions
The transformation of these gases into acids occurs through a series of oxidation reactions in the atmosphere. These reactions often involve the presence of oxygen (O2) and water vapour (H2O).
Nitrogen oxides (NOx):
NO is oxidised to NO2.
NO2 reacts further with oxygen and water to form nitric acid (HNO3).
Sulphur dioxide (SO2):
SO2 reacts with oxygen to form sulphur trioxide (SO3).
SO3 dissolves in water, producing sulphuric acid (H2SO4).
Formation of Nitric Acid (HNO3):
2NO2 + H2O → HNO3 + HNO2
NO2 = Nitrogen dioxide (gas)
H2O = Water vapour
HNO3 = Nitric acid (aqueous)
HNO2 = Nitrous acid (aqueous, less stable)
Following this, nitric acid readily dissolves into cloud droplets, increasing rainfall acidity.
Sulphur Pathways
The conversion of sulphur dioxide is particularly important in acid rain chemistry. The process may be catalysed by particulate matter in the atmosphere.
Formation of Sulphuric Acid (H2SO4):
SO2 + O2 → SO3
SO3 + H2O → H2SO4
SO2 = Sulphur dioxide (gas)
O2 = Oxygen (gas)
SO3 = Sulphur trioxide (gas)
H2O = Water vapour
H2SO4 = Sulphuric acid (aqueous)
This acid is highly soluble, readily mixing with atmospheric moisture to create acid precipitation.
Atmospheric Processes in Acid Formation
Role of Clouds and Rainfall
The formation of acid rain requires the mixing of acidic gases with cloud droplets or precipitation. Clouds act as natural reactors where:
Gaseous pollutants dissolve into water droplets.
Chemical reactions accelerate under high humidity.
Acids accumulate until released as precipitation.
This makes areas with frequent rainfall particularly vulnerable to acid deposition.
Photochemical Influence
Sunlight can accelerate the oxidation processes, especially in urban regions with elevated NOx concentrations. Ultraviolet light increases reaction rates, enhancing the production of nitric acid.
Pathways of Deposition
Wet Deposition
This occurs when acidic compounds dissolve in rain, snow, sleet, or fog and fall to the Earth’s surface.
Produces acid rain with pH values typically below 5.6.
Strongly influenced by weather patterns and precipitation frequency.
Dry Deposition
Acidic gases and particulates can also settle directly on surfaces without precipitation. Later, when these compounds mix with water, they form acids, further contributing to environmental harm.
Both wet deposition and dry deposition processes are important for understanding global distribution.
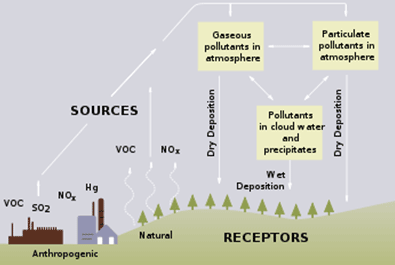
Cycle diagram contrasting wet and dry deposition within the atmosphere–surface exchange, tying directly to acid rain formation products (H₂SO₄ and HNO₃). Labels show how acids formed aloft are delivered by precipitation or settle directly as gases/particles. Minor references to ecosystem effects extend beyond formation but help situate the pathways. Source.
Spatial and Temporal Distribution
Regional Transport
Acid rain formation is not confined to emission sites. Winds carry SO2 and NOx across regions, producing transboundary environmental effects. For example, emissions in one country may cause acid rain damage in neighbouring regions.
Timescales of Reaction
Some reactions, such as NO2 dissolving in water, occur relatively quickly.
Others, like SO2 oxidation to SO3, can take longer, particularly in dry conditions.
This variation explains why acid rain can occur close to or far from emission sources.
Factors Influencing Acid Rain Formation
Human Activities
Coal-fired power plants: Major sources of SO2.
Vehicle emissions: Key contributors of NOx.
Industrial processes: Smelting and oil refining increase pollutant output.
Natural Contributions
Volcanoes release large amounts of SO2.
Wildfires and lightning strikes contribute to NOx.
Although significant, these natural sources are overshadowed by human industrial activity.
Key Points for IB ESS Students
Acid deposition originates primarily from the atmospheric transformation of NOx and SO2.
The essential reactions involve oxidation and hydration, producing nitric and sulphuric acids.
Both wet deposition and dry deposition processes are important for understanding global distribution.
Human activity remains the dominant cause, though natural events play secondary roles.
FAQ
Free radicals, such as hydroxyl radicals (OH•), act as atmospheric catalysts that speed up the oxidation of sulphur dioxide and nitrogen oxides.
They react with SO2 to form sulphate particles and accelerate the conversion of NO2 into nitric acid. Without these radicals, the chemical transformations would occur much more slowly, limiting the intensity of acid rain.
Pollutants such as SO2 and NOx can remain in the atmosphere for several days before reacting with oxygen and water.
During this time, they are transported by prevailing winds across large distances. This leads to acid rain in regions far from industrial centres, making acid deposition a transboundary issue.
Temperature affects the speed of chemical reactions in the atmosphere.
Higher temperatures increase the rate of oxidation of NOx and SO2.
Warmer air also promotes the formation of photochemical oxidants, enhancing nitric acid production.
In colder climates, slower reactions can reduce formation but snow and ice may trap acidic compounds, later releasing them during melt.
In-cloud reactions occur within cloud droplets where gases dissolve and transform into acids before falling as precipitation.
Below-cloud reactions occur when falling raindrops absorb acidic gases or particles from the air as they descend. Both processes contribute to overall acidity, but in-cloud reactions are often the dominant mechanism.
Volcanoes release large amounts of sulphur dioxide directly into the atmosphere, sometimes injecting gases into the stratosphere.
Unlike human sources, volcanic emissions can create short-term, highly localised acid rain events. However, the long-term, continuous contribution from burning fossil fuels is far more significant on a global scale.
Practice Questions
Question 1 (2 marks)
Identify the two main atmospheric gases that combine with oxygen and water to form acids responsible for acid rain.
Mark scheme:
1 mark for correctly identifying nitrogen oxides (NOx).
1 mark for correctly identifying sulphur dioxide (SO2).
Question 2 (5 marks)
Explain how nitrogen oxides and sulphur dioxide are transformed in the atmosphere into acids that contribute to acid rain.
Mark scheme:
1 mark for stating that both gases undergo oxidation reactions.
1 mark for describing the conversion of NO to NO2 and subsequent reaction with water/oxygen to form nitric acid (HNO3).
1 mark for describing the conversion of SO2 to SO3.
1 mark for stating that SO3 dissolves in water to form sulphuric acid (H2SO4).
1 mark for linking these processes to acid deposition in rainfall or wet/dry deposition pathways.

