OCR Specification focus:
‘Select and apply appropriate units for measurements, and convert between units accurately when necessary.’
Measuring biological variables reliably depends on selecting correct units and performing conversions accurately so data remain comparable, valid and clearly communicated between scientists and experiments.
The importance of measurement units in biology
Accurate measurement is essential for reliable data, valid comparisons and meaningful analysis. Using standard units enables scientists to communicate results consistently and avoid misinterpretation between laboratories and countries. Biological investigations require students to work confidently with units across mass, volume, temperature, concentration, time and length, ensuring appropriate SI units are applied whenever possible. SI units are internationally recognised and reduce confusion when collecting and presenting raw data.
SI units and their correct use
SI units are the globally accepted System of International Units, used to ensure clarity and consistency in scientific measurements.
Illustration of the seven SI base units. These foundational units support all derived measurements used later in biological calculations and data processing. Source.
The most common SI units in A-Level Biology include metres (m) for length, kilograms (kg) for mass, and seconds (s) for time. Units must always be written clearly, using lower-case letters except where a unit is named after a person, such as degrees Celsius (°C) or Pascal (Pa).
SI Unit: The internationally agreed standard unit for measuring physical quantities to allow universal communication of scientific data.
Biology also uses derived units, which are combinations of base units. For example, concentration is commonly expressed as mol dm⁻³ and rate as cm³ s⁻¹. Even when units are derived, they must remain consistent throughout data collection and processing.
Unit prefixes and scientific notation
Prefix symbols indicate multiples or fractions of SI units. In biology, common prefixes help express microscopic and macroscopic scales clearly. Frequently used prefixes include:
kilo- (k) = 10³
centi- (c) = 10⁻²
milli- (m) = 10⁻³
micro- (µ) = 10⁻
nano- (n) = 10⁻⁹
Using prefixes avoids long strings of zeros and improves readability of measurements. For extremely large or small values, scientific notation is also essential for clarity.
Converting between units
Students must convert unit prefixes accurately to maintain data integrity. Conversions rely on multiplying or dividing by powers of ten. For example, converting from millimetres to metres involves dividing by 1000 because 1 mm = 10⁻³ m. Biological investigations often require conversions between cm, mm, µm and nm for microscopy, or between cm³, dm³ and litres for volumes.
EQUATION
—-----------------------------------------------------------------
Unit Conversion (new value) = original value × conversion factor
Conversion factor = numerical multiplier that links one unit to another
—-----------------------------------------------------------------
Unit conversions also apply when calculating concentration, volume or rate, ensuring processed data matches the units used in tables, graphs and equations. Consistency is essential because mixing units can distort results and invalidate conclusions.
A useful strategy is to write out the unit pathway step-by-step, ensuring each conversion is completed without skipping stages. This reduces errors and reinforces good experimental technique.
Choosing appropriate units
To meet specification expectations, students must select the most suitable unit for the scale and nature of the measurement. Appropriate unit choice prevents loss of precision and improves the usefulness of recorded data. Suitable units should:
Match the magnitude of the quantity
Allow appropriate significant figures to be used
Support straightforward graphing and comparison
Follow SI conventions wherever possible
For example, recording a plant shoot as 0.00012 m is inappropriate; 0.12 mm or 120 µm is clearer and avoids unnecessary zeros. Likewise, gas production in a practical is clearer in cm³ s⁻¹ than m³ s⁻¹.
Units in experimental recording and presentation
Measurements must always include their units in headings, labels and data tables. A measurement without a unit is biologically meaningless. When repeating measurements, units should appear once in the heading rather than after every number in a column, preventing clutter and improving clarity.
When plotting graphs, both axes must include the variable name and unit, such as Rate of reaction (cm³ s⁻¹). Unit consistency becomes especially important during data processing, when students calculate means, gradients or rates using raw observations.
Common measurement contexts in A-Level Biology
Students will routinely apply and convert units when measuring:
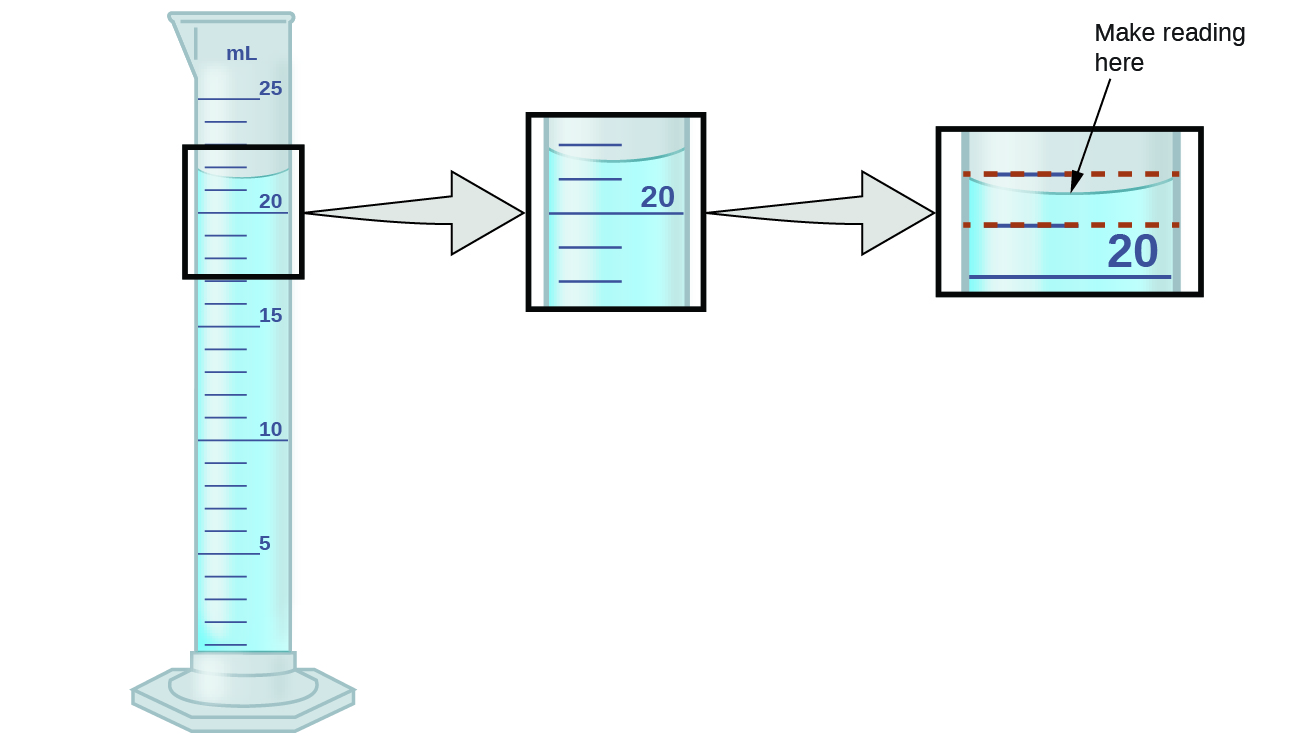
Diagram showing the correct meniscus reading at the lowest point of the curve. Accurate volume readings ensure unit-correct data for later conversions used in biological investigations. Source.
Microscopy scales (mm, µm, nm)
Volumes of liquids (cm³, dm³, dm³ mol⁻¹)
Concentrations (mol dm⁻³ or g dm⁻³)
Temperature (°C or K)
Time (s, min, h
Rates (cm³ s⁻¹, s⁻¹, min⁻¹)
Avoiding unit errors is vital, as mis-scaled or inconsistent measurements propagate through calculations and damage reliability. Skilled unit handling supports precision and ensures results meet assessment criteria for valid scientific data.
FAQ
SI units allow scientists to compare results globally without reinterpreting measurements. This avoids confusion caused by informal units such as inches or gallons.
They also support derived units used in biology, such as mol dm-3 or cm3 s-1, enabling straightforward data processing and reducing the risk of conversion errors.
Accuracy refers to whether the unit and scale chosen reflect the true size of what is being measured, such as using micrometres instead of centimetres for cells.
Precision refers to how detailed the measurement is, often linked to the number of decimal places or graduations on equipment. Measurements can be precise without being accurate if the wrong unit is used.
Scientific notation is most useful when numbers are extremely large or small, especially when repeated conversion steps would be time-consuming.
It is often preferred in calculations involving multiple measurements because powers of ten are easier to manipulate than long decimal values. This speeds up processing and reduces rounding errors.
Choose units that match the scale of the variable being measured. For example:
• µm or nm for cells and organelles
• cm3 or dm3 for liquid volumes
Avoid units that create unnecessary zeros, and select units that will remain practical when calculating rates or plotting graphs.
Stating the unit removes ambiguity and ensures anyone reading the data can interpret the values correctly without assumptions.
It also matches scientific conventions for data presentation, helping examiners and researchers verify that the correct scale and conversions have been used before analysing results.
Practice Questions
Question 1 (2 marks)
A student measures the diameter of an onion epidermis cell as 85 micrometres. Convert this measurement into millimetres. Show your working.
Question 1 (2 marks)
1 mark for correct conversion factor:
• 1 micrometre = 0.001 millimetres or 1 micrometre = 10⁻³ millimetres
1 mark for correct answer:
• 85 micrometres = 0.085 millimetres
Question 2 (5 marks)
A student investigates the rate of gas production in yeast. The gas volume is recorded in cm3, while time is recorded in minutes.
Explain why it is important to use appropriate units and consistent unit conversions when processing and presenting the results. In your answer, refer to scientific communication, data processing, and the reliability of conclusions.
Question 2 (5 marks)
Award 1 mark for each valid point.
• Using standard SI or derived units allows data to be compared between investigations (1)
• Consistent unit use prevents errors in calculations such as means, rates or gradients (1)
• Mixing units (e.g., cm3 and mm3 or minutes and seconds) can lead to incorrect analysis or invalid conclusions (1)
• Units must be stated clearly in tables and on graph axes for effective scientific communication (1)
• Correct unit conversions ensure patterns, trends and relationships shown by data are reliable (1)

