OCR Specification focus:
‘Investigate effects of temperature and solvents on membrane structure and permeability.’
Cell membranes act as dynamic barriers controlling substance exchange. Their permeability changes with temperature and solvent exposure, affecting essential cellular processes and stability.
Understanding Membrane Permeability
Biological membranes are partially permeable structures composed mainly of phospholipids, proteins, cholesterol, and carbohydrates. Permeability refers to how easily substances cross this barrier, influenced by both environmental and chemical factors.
The Fluid Mosaic Model
The fluid mosaic model describes membranes as fluid layers of phospholipid molecules with embedded proteins and cholesterol, giving flexibility and selective permeability.
Fluid Mosaic Model: A model describing the cell membrane as a dynamic phospholipid bilayer with floating proteins, allowing movement and selective permeability.
Phospholipids have hydrophilic heads and hydrophobic tails, forming a bilayer.
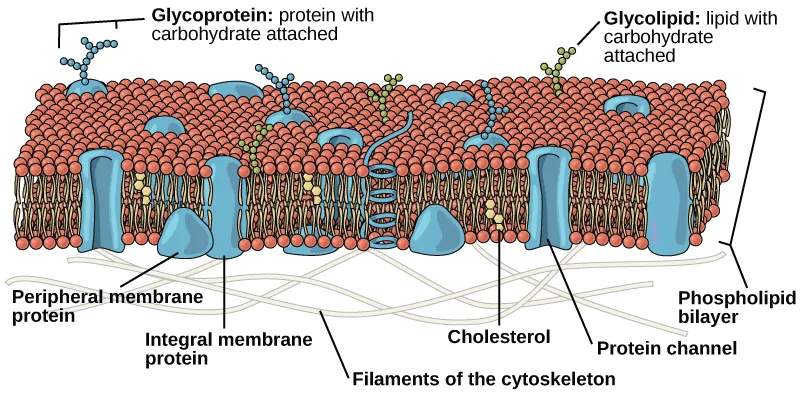
A labelled diagram of the plasma membrane illustrating phospholipids, integral and peripheral proteins, carbohydrates, and cholesterol within a fluid mosaic bilayer. This structure explains why heat can increase lipid movement and protein denaturation, and why organic solvents disrupt the hydrophobic core, both raising permeability. The figure includes components (e.g., carbohydrate chains) beyond the OCR focus, but they are standard features of the model and do not add procedural complexity. Source.
This structure ensures hydrophilic molecules (e.g., ions, polar molecules) are largely excluded, while non-polar molecules (e.g., oxygen, carbon dioxide) diffuse freely.
Factors Affecting Membrane Permeability
Two key experimental variables profoundly influence membrane structure and permeability: temperature and solvents.
Temperature and Membrane Permeability
Temperature affects both the kinetic energy of molecules and the integrity of the phospholipid bilayer.
Low Temperatures
Reduced kinetic energy decreases molecular motion.
Phospholipids become tightly packed, reducing membrane fluidity.
Transport of molecules slows; permeability is low.
Moderate Temperatures (Physiological Range)
Increased kinetic energy enhances phospholipid movement, maintaining an optimal level of fluidity.
Enzymes and carrier proteins embedded in the membrane function efficiently.
High Temperatures
Excessive heat increases kinetic energy, causing phospholipids to move apart.
Proteins denature, altering shape and forming gaps in the bilayer.
Permeability rises sharply, allowing leakage of substances such as pigments, ions, or enzymes.
This principle is demonstrated through beetroot membrane experiments, where increased pigment leakage (betacyanin) indicates higher permeability at elevated temperatures.
The Temperature Coefficient (Q10)
EQUATION
—-----------------------------------------------------------------
Temperature Coefficient (Q10) = Rate at (T + 10°C) / Rate at T°C
Q10 = Dimensionless value indicating how reaction rate changes with a 10°C temperature rise.
—-----------------------------------------------------------------
Although typically applied to enzyme reactions, Q10 can indirectly describe temperature effects on diffusion and membrane permeability — usually doubling until structural damage occurs.
Solvents and Membrane Integrity
Organic solvents, such as ethanol or acetone, also influence membrane permeability by dissolving lipids or disrupting protein structure.
Mechanism of Solvent Action
Non-polar solvents interact with hydrophobic fatty acid tails, causing membrane lipids to dissolve or separate.
This leads to loss of structural integrity and increased permeability.
Polar solvents (like water) are less disruptive, as they interact favourably with hydrophilic heads rather than the hydrophobic core.
Solvent Concentration and Type
Low concentrations of ethanol may fluidise membranes moderately.
High concentrations can cause complete disruption, releasing intracellular contents.
Acetone acts even more rapidly than ethanol, making it an effective demonstration of solvent effects.
Biological Relevance
Cells exposed to alcohols or detergents suffer membrane destabilisation, affecting functions such as ion regulation and metabolic control. This principle explains antiseptic properties of alcohol and solvent-induced cell lysis.
Practical Investigations into Membrane Permeability
The OCR specification emphasises practical investigations into how temperature and solvents affect permeability. Such experiments develop key practical and analytical skills.
Common Experimental Model: Beetroot Membranes
Beetroot cells contain vacuoles filled with betalain pigment, retained by the tonoplast and plasma membranes. When these membranes are damaged, pigment leaks into the surrounding solution, providing a visible indicator of permeability changes.
Step-by-Step Process
Cut equal-sized beetroot discs using a cork borer.
Wash to remove excess surface pigment.
Place discs in test tubes with equal volumes of water or solvent.
Expose samples to a range of temperatures (e.g., 0°C to 70°C).
After incubation, measure pigment release using a colorimeter.

A cuvette inserted in a bench-top spectrophotometer used for colorimetry. Measuring absorbance of leaked pigment provides an objective readout of membrane permeability following temperature or solvent exposure. The photo shows general spectrophotometer use (not beetroot specifically), but the apparatus is exactly the one specified for the OCR-level practical. Source.
Colorimeter: An instrument that measures the absorbance or transmission of light through a coloured solution to quantify concentration.
Higher absorbance values indicate greater membrane damage and therefore higher permeability.
Control Variables and Data Accuracy
To ensure reliable results:
Use equal beetroot surface areas and volumes.
Maintain constant exposure times.
Calibrate colorimeters and use blank controls (distilled water).
Repeat readings for replication and averaging.
Consistent methodology allows quantitative comparisons of membrane stability under different conditions.
Interpretation of Data
Temperature Effects
Gradual increase in absorbance with temperature rise indicates progressive membrane destabilisation.
A sharp spike near 60–70°C reflects protein denaturation and membrane rupture.
Solvent Effects
Increasing ethanol or acetone concentration correlates with greater pigment leakage.
The rate of change reveals how different solvents vary in their disruptive capacity.
Analytical Extension: Linking to Membrane Composition
Cholesterol in animal membranes reduces fluidity fluctuations, making membranes more resistant to temperature changes.
Plant and bacterial membranes, lacking cholesterol, are more vulnerable to extreme conditions.
Variation in fatty acid saturation influences membrane stability: more unsaturated lipids increase fluidity, enhancing temperature tolerance.
Safety and Ethical Considerations
Handle solvents like ethanol and acetone in well-ventilated areas and away from flames.
Use protective equipment (gloves, goggles).
Dispose of organic solvents following laboratory safety protocols.
Ethically, the beetroot model avoids animal use while demonstrating real biological effects.
Linking Permeability to Cellular Function
Altered membrane permeability affects:
Nutrient and ion balance
Signal transduction through membrane receptors
Enzyme activity within embedded proteins
Thus, maintaining optimal temperature and solvent conditions is vital for cell survival and homeostasis.
FAQ
Phospholipids with unsaturated fatty acid tails contain double bonds that create kinks, preventing tight packing. This increases membrane fluidity and permeability, especially at lower temperatures.
In contrast, saturated fatty acids pack more closely, making membranes more rigid and less permeable.
The ratio of saturated to unsaturated lipids determines how susceptible a membrane is to temperature-induced damage.
Beetroot cells contain betacyanin pigment stored in the vacuole, which does not normally leave intact cells. When membranes are damaged by heat or solvents, the pigment leaks out, visibly colouring the surrounding solution.
This provides a convenient and quantifiable way to assess membrane damage using colorimetry.
Beetroot is also safe, inexpensive, and easily standardised for school and college experiments.
Ethanol interacts with the hydrophobic tails of phospholipids, reducing the hydrophobic interactions that stabilise the bilayer.
This causes lipids to become disordered, increasing fluidity and permeability.
At higher concentrations, ethanol can denature membrane proteins, creating pores that allow substances to leak through.
At very low temperatures, ice crystals may form and puncture membranes, causing irreversible damage.
Additionally, when ice forms, extracellular solute concentration rises, leading to osmotic imbalance and cell dehydration.
Upon thawing, membranes become leaky as lipids and proteins are displaced or damaged by previous freezing.
It only measures pigment concentration, not the type or extent of molecular damage.
Uneven beetroot surface area or thickness can affect consistency.
Overlapping absorbance spectra may reduce accuracy if pigment degradation occurs.
To improve precision, use standard curves, maintain constant lighting, and ensure identical cuvette conditions during measurement.
Practice Questions
Question 1 (2 marks)
Explain why increasing temperature above 60°C leads to an increase in cell membrane permeability.
Mark Scheme:
1 mark for stating that high temperatures cause phospholipids to move apart or increase fluidity, disrupting membrane structure.
1 mark for stating that membrane proteins denature, creating gaps that allow substances to leak through.
Question 2 (5 marks)
A student investigates the effect of different ethanol concentrations on the permeability of beetroot cell membranes.
Describe how the student could carry out this investigation and explain how the results would be used to assess membrane permeability.
Mark Scheme:
1 mark for describing the use of equal-sized beetroot discs to ensure a fair test.
1 mark for stating that discs should be placed in different ethanol concentrations (e.g. 0%, 20%, 40%, etc.) for a set time period.
1 mark for stating that leaked pigment in the surrounding solution is measured using a colorimeter.
1 mark for explaining that absorbance increases as more pigment leaks, indicating higher membrane permeability.
1 mark for explaining the need to control variables (e.g. temperature, time, beetroot size) to ensure reliable and valid results.

