OCR Specification focus:
‘Explain osmosis via water potential gradients and effects on plant and animal cells.’
Osmosis is a fundamental biological process vital for maintaining cellular stability and organismal homeostasis. It involves the movement of water across partially permeable membranes, driven by differences in water potential, influencing both plant and animal cell behaviour.
Understanding Osmosis
Definition and Core Principle
Osmosis: The passive movement of water molecules from a region of higher water potential to a region of lower water potential, across a partially permeable membrane.
This process requires no metabolic energy (ATP), as it relies solely on the kinetic energy of water molecules. Osmosis underpins key physiological activities such as nutrient absorption, turgor maintenance, and cellular volume regulation.
Water Potential (Ψ)
Concept and Definition
Water Potential (Ψ): The measure of the tendency of water to move from one area to another, determined by solute concentration and pressure; measured in kilopascals (kPa).
Pure water has the highest water potential, defined as 0 kPa. The addition of solutes lowers water potential, making Ψ more negative. Water therefore moves down a water potential gradient, from higher (less negative) to lower (more negative) values.
Components of Water Potential
Water potential consists of two main components:
Solute potential (Ψs) – also called osmotic potential, representing the effect of solute concentration on water movement. Increasing solute concentration lowers Ψs (more negative).
Pressure potential (Ψp) – the physical pressure exerted on water within cells. In plant cells, this results from the cell wall resisting expansion, leading to turgor pressure.
EQUATION
—-----------------------------------------------------------------
Water Potential (Ψ) = Solute Potential (Ψs) + Pressure Potential (Ψp)
Ψ = Ψs + Ψp
Ψ = Total water potential (kPa)
Ψs = Solute potential (kPa; always ≤ 0)
Ψp = Pressure potential (kPa; ≥ 0 in turgid cells)
—-----------------------------------------------------------------
This equation explains how internal and external conditions determine net water movement across membranes.
Osmosis Across Cell Membranes
The Partially Permeable Membrane
Cell membranes are partially permeable, allowing small polar molecules like water to pass through but restricting ions and larger solutes. Movement occurs primarily via:
Diffusion through phospholipid bilayers (slow process).
Facilitated diffusion through aquaporins, which are channel proteins specialised for water transport.
Osmotic Gradients
An osmotic gradient exists when two regions have different water potentials. Water moves down the gradient, and the net direction of movement depends on solute concentration differences across the membrane.
Osmosis in Animal Cells
Behaviour in Different Solutions
Animal cells, such as red blood cells, lack cell walls and are highly sensitive to osmotic changes:
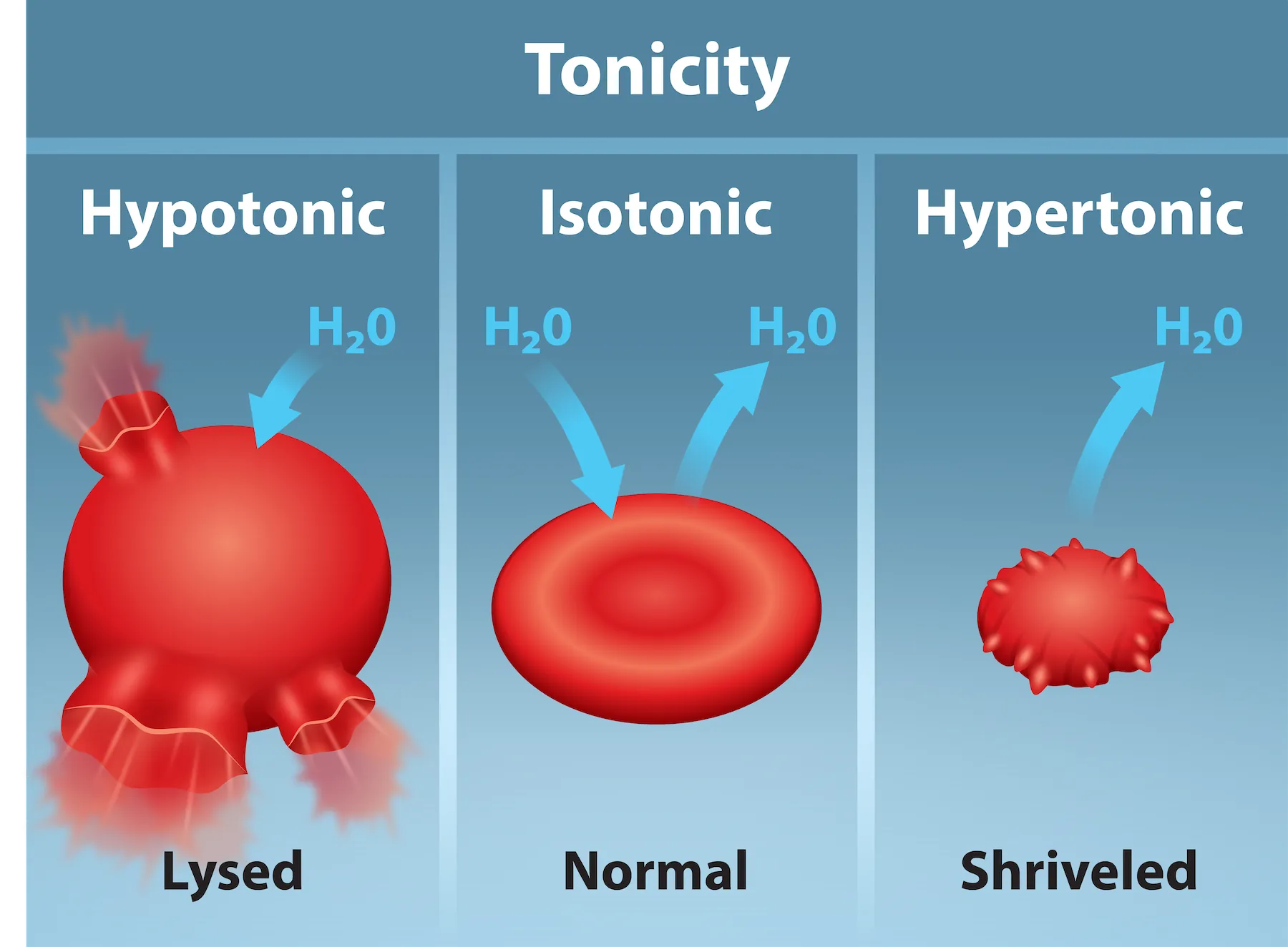
This textbook figure shows red blood cells in hypotonic, isotonic, and hypertonic solutions, illustrating haemolysis, a normal biconcave form, and crenation, respectively. It aligns with the concept of water moving down its water potential gradient across a partially permeable membrane. The figure includes brief labels only; it does not add concepts beyond the syllabus focus. Source.
Hypotonic solution (higher Ψ outside cell):
Water enters the cell by osmosis. Excessive influx causes cell swelling and lysis (bursting).
Example: red blood cells may haemolyse in pure water.Isotonic solution (equal Ψ inside and outside):
No net water movement occurs. The cell maintains its normal shape and volume, achieving osmotic equilibrium.Hypertonic solution (lower Ψ outside cell):
Water leaves the cell by osmosis, causing cell shrinkage or crenation.
These osmotic responses are critical for understanding homeostatic mechanisms in animals, such as kidney regulation and blood plasma osmolarity.
Osmosis in Plant Cells
Plant Cell Adaptations
Plant cells possess rigid cellulose cell walls, providing structural support and resistance to excessive expansion. This influences their osmotic behaviour significantly.
Effects of Water Movement
Hypotonic environment (higher Ψ outside):
Water enters the cell via osmosis. The vacuole enlarges, the plasma membrane presses against the cell wall, and the cell becomes turgid.
Turgidity supports plant structure, maintaining upright stems and leaves.
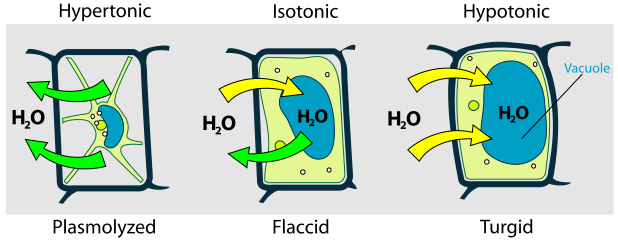
Labeled plant cells illustrate how water potential (Ψ) differences drive osmosis to produce turgid, incipiently plasmolysed, and plasmolysed states. The diagram highlights the role of pressure potential (Ψp) from the cell wall in resisting further expansion. Extra contextual labels (e.g., arrows for water movement) are included but remain directly relevant. Source.
Isotonic environment (equal Ψ):
No net water movement occurs. The cell is described as incipiently plasmolysed, meaning internal pressure is lost but the membrane remains attached to the cell wall.Hypertonic environment (lower Ψ outside):
Water exits the cell via osmosis. The vacuole shrinks, and the plasma membrane pulls away from the cell wall — a process called plasmolysis.
A red onion epidermal cell in a hypertonic NaCl solution shows the protoplast retracted from the cell wall as water leaves the cell. This is plasmolysis, resulting in loss of turgor and a flaccid state. The photo’s coloration and texture reflect natural pigments and optics from light microscopy. Source.
Plasmolysed cells become flaccid, leading to wilting in plant tissues.
Turgor Pressure and Plant Support
Turgor Pressure: The hydrostatic pressure exerted by the cell contents against the cell wall, providing structural support and resistance to further water uptake.
Turgor is essential for mechanical strength and growth in plants. The interplay between water potential and turgor pressure determines cell expansion during development.
Water Potential Gradients in Organisms
Importance of Gradients
Cells rely on carefully maintained water potential gradients for:
Nutrient and ion transport via xylem and phloem.
Maintenance of blood and tissue fluid osmolarity.
Regulation of stomatal aperture through guard cell turgor changes.
In multicellular organisms, osmoregulation—the control of water and solute balance—is coordinated by specialised organs like kidneys in animals and stomata in plants.
Osmosis in Real Biological Contexts
Root Hair Cells:
Absorb water from soil with higher Ψ compared to their cytoplasm. This initiates the transpiration stream, transporting water to leaves.Guard Cells:
Gain water during photosynthesis when Ψ is higher outside, becoming turgid and opening stomata to allow gas exchange. When Ψ decreases, they lose water, become flaccid, and close stomata to conserve water.Animal Cells in Body Fluids:
Must remain isotonic with surrounding fluids to prevent osmotic damage, achieved through homeostatic regulation of solute concentrations.
Investigating Osmosis
Experimental Approaches
Students can investigate osmosis by:
Measuring mass or length changes in plant tissue (e.g. potato cylinders) immersed in sucrose solutions of varying concentration.
Plotting percentage change against solution concentration to estimate the solute potential where Ψcell = Ψsolution (no net water movement).
Practical Significance
These experiments reinforce understanding of:
The directionality of water movement.
The relationship between solute concentration and water potential.
The physiological consequences of osmotic imbalance in biological systems.
FAQ
Water potential depends not only on solute concentration but also on pressure potential (Ψp). In plant cells, the rigid cell wall exerts a positive pressure that increases Ψ.
Temperature and matrix effects (binding of water to surfaces such as cell walls) can also slightly alter Ψ, although these are less significant in most biological contexts.
Aquaporins are specialised channel proteins embedded in cell membranes that allow rapid, selective water movement.
They open or close in response to hormonal or osmotic signals, such as antidiuretic hormone (ADH) in animal kidneys.
By altering aquaporin abundance, cells can regulate membrane permeability to water, maintaining homeostasis without changing solute concentrations.
Once severe plasmolysis occurs, the plasma membrane may detach irreversibly from the cell wall and suffer structural damage.
This prevents normal reabsorption of water and may lead to loss of metabolic function.
In contrast, cells experiencing incipient plasmolysis (mild water loss) can regain turgor when returned to a hypotonic environment because membrane–wall contact remains mostly intact.
Guard cells surrounding stomata control pore opening by changing their turgor pressure through osmosis.
When water enters guard cells (higher external Ψ), they become turgid, curving apart to open the pore.
When water leaves (lower external Ψ), they become flaccid, and the pore closes.
This process is driven by ion transport, particularly of potassium ions, which alters solute potential and thus water potential inside the guard cells.
Osmotic stress disrupts cytoplasmic water content, which alters enzyme conformation and substrate diffusion rates.
In hypertonic conditions, enzymes may denature or slow down due to dehydration and increased ionic strength.
In hypotonic conditions, excessive water uptake can dilute enzymes and substrates, reducing reaction efficiency.
Cells therefore rely on osmotic regulation mechanisms (e.g. compatible solutes) to preserve optimal enzyme activity.
Practice Questions
Question 1 (2 marks)
Define osmosis and describe the direction of water movement in terms of water potential.
Mark scheme:
1 mark: Correct definition of osmosis as the passive movement of water molecules through a partially permeable membrane.
1 mark: States that water moves from a region of higher (less negative) water potential to a region of lower (more negative) water potential.
Question 2 (5 marks)
Describe and explain the effects of placing a plant cell and an animal cell in a solution with a lower water potential than their cytoplasm.
Mark scheme:
1 mark: States that water leaves both cells by osmosis.
1 mark: Explains that this occurs because the external solution has a lower (more negative) water potential than the cytoplasm.
1 mark: States that the plant cell becomes flaccid or plasmolysed as the plasma membrane pulls away from the cell wall.
1 mark: States that the animal cell shrinks or becomes crenated due to loss of water.
1 mark: Explains that animal cells lack a cell wall, so they cannot resist water loss and therefore shrink more drastically.

