OCR Specification focus:
'Select appropriate apparatus, equipment and techniques that match the experimental aims and conditions for reliable data collection and analysis.'
In practical chemistry, selecting the right apparatus and techniques is essential to ensure accurate data collection and meaningful analysis. This subsubtopic focuses on how to make these decisions effectively, tailored to the experiment's aims.
Apparatus and Techniques Selection
When planning a chemistry experiment, one of the most critical steps is selecting the appropriate apparatus and techniques. The goal is to ensure that the experiment is set up to collect reliable data and that the conditions are controlled to avoid errors. This process requires a deep understanding of both the theoretical aspects of the experiment and the available practical tools.
Understanding Experimental Aims
The first step in selecting apparatus and techniques is to understand the experimental aims. The apparatus and techniques chosen must align directly with the scientific objectives of the experiment. For example, if the aim is to measure the concentration of a solution, the selection of a titration apparatus and burettes would be appropriate.
Experimental aims: Clearly defined objectives of the experiment.
Apparatus choice: Must match the requirements of the experiment for accurate data collection.
Once the aims are clear, you can proceed to choose the most suitable tools and methods for gathering the necessary data.
Apparatus Selection Criteria
Selecting the correct apparatus involves considering various factors, such as the quantity of the substance to be measured, the precision required, and the type of measurements to be taken. The right equipment will directly influence the accuracy and reliability of the results.
Quantity of substance: Select apparatus capable of handling the volume or mass required for the experiment. For instance, a 50 cm³ burette may be more suitable for smaller volumes, whereas a 100 cm³ might be needed for larger titrations.
Precision: Apparatus should allow for the precision necessary for the experiment. For example, a digital balance would be required when high precision is needed, as opposed to a regular balance that might have a higher margin of error.
Type of measurement: Choose equipment that aligns with the nature of the measurement. For measuring temperature, a thermometer or thermocouple might be used, depending on the range required. For volume measurement, a pipette or burette would be chosen based on the amount and precision needed.
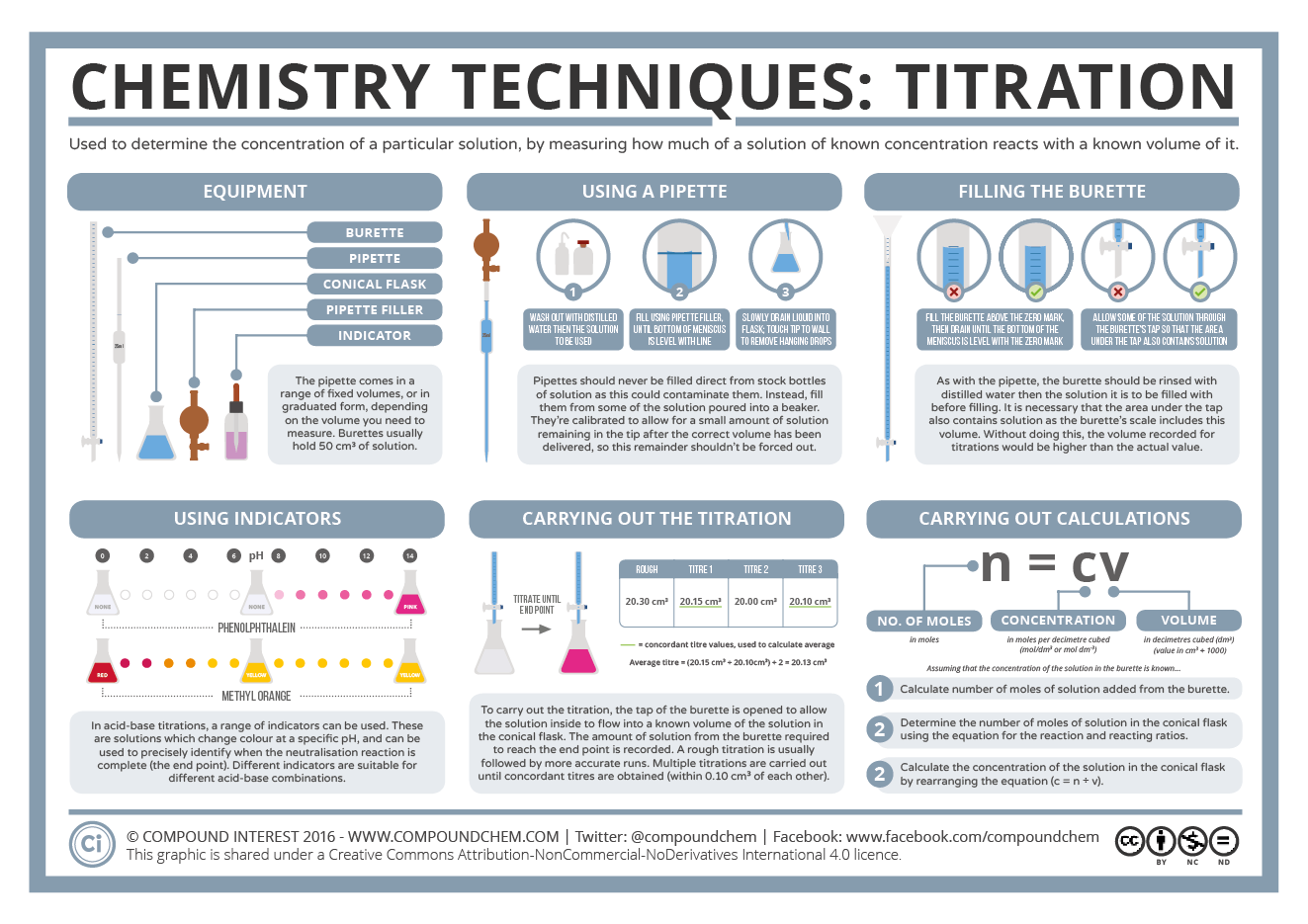
This diagram compares three common laboratory tools for volume measurement. The burette, pipette, and measuring cylinder are labelled for comparison in terms of their precision and suitability for different tasks. Source
Techniques Selection Criteria
Selecting the correct techniques is as important as choosing the right apparatus. Techniques refer to the methods of performing the experiment and should be suited to the materials and aims of the experiment.
Accuracy: Techniques such as titration or gravimetric analysis may be selected for high-accuracy measurements, depending on the experimental goals. Ensure the technique can deliver results within the acceptable error margin.
Data analysis: Some techniques may generate qualitative data, while others provide quantitative results. For example, colorimetry might be selected for analyzing absorbance in a solution, while spectrophotometry is often used for more precise measurements.
Time constraints: Some techniques are time-consuming, such as distillation, while others, like precipitation reactions, might be quicker but may not yield the same level of precision.
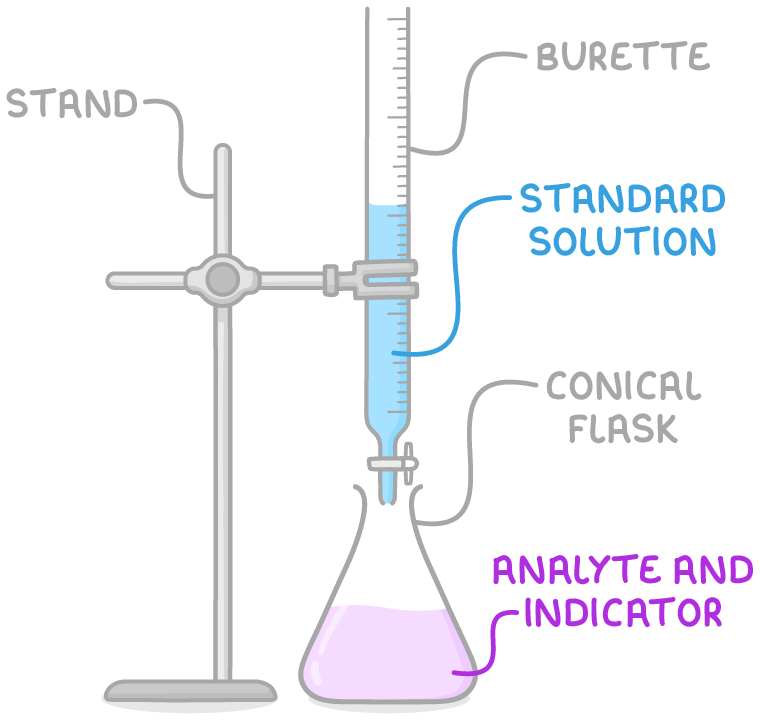
This image shows the set-up for a typical acid-base titration. The burette, pipette, and flask are shown with their respective roles in determining the volume of titrant and analyte in the reaction. Source
Matching Apparatus with Experimental Conditions
Once the correct apparatus and techniques are chosen, it's important to match them with the conditions of the experiment. This includes factors like temperature, pressure, and the nature of the substances involved.
Temperature control: Some experiments, like those involving endothermic or exothermic reactions, may require precise temperature control, necessitating the use of equipment like thermostats or water baths.
Pressure control: Experiments involving gaseous substances or reactions in a sealed container may need pressure vessels or vacuum pumps to control the environment.
Reactivity of substances: For experiments with reactive or toxic substances, select apparatus that ensures safety, such as fume hoods or gas-tight containers.
Ensuring Reliable Data Collection
The reliability of experimental data is directly affected by the choice of apparatus and techniques. Ensure that the selected equipment is well-maintained, clean, and in good working order to avoid inaccuracies. For instance, pipettes should be checked for calibration, and burettes should be inspected for leaks.
Calibration: Regularly calibrate measuring instruments to ensure their accuracy. This is particularly important for instruments like pH meters, spectrometers, or balances.
Condition of equipment: All equipment must be free from contaminants that could skew results. Cleaning apparatus thoroughly before use is essential, especially when working with reactive substances or solvents.
FAQ
When selecting apparatus for a titration, consider:
The accuracy required in measuring the titrant volume (e.g., use a burette).
The precision needed for measuring the acid volume (e.g., use a pipette).
The size of the reaction vessel, ensuring a conical flask is suitable for swirling without spilling.
The nature of the solution being used, ensuring compatibility with the apparatus material (e.g., glassware for corrosive substances).
The selected technique impacts:
Data reliability: Using the wrong technique can introduce significant errors, such as using a rough method when precision is needed.
Accuracy: Some techniques are better suited for high-accuracy measurements, such as gravimetric analysis versus volumetric analysis.
Time efficiency: Some methods are faster but less precise, while others may require more time for greater accuracy.
A burette is ideal for titrations because it:
Allows for precise measurement of small volumes of titrant.
Can be easily controlled, enabling gradual addition of titrant to the analyte.
Provides clear and accurate readings, essential for determining the endpoint of the titration.
When selecting apparatus:
Ensure all glassware is free from cracks and chips, which could lead to breakage during use.
Use fume hoods or other containment methods when working with volatile or toxic substances.
Ensure that any apparatus exposed to high temperatures or pressures is rated for those conditions to prevent accidents.
Calibration ensures:
Accuracy: Measuring instruments give accurate results, minimising systematic errors.
Consistency: It ensures that measurements are consistent across different experiments and setups.
Confidence: Properly calibrated equipment leads to reliable data, which is crucial for drawing valid conclusions from experimental results.
Practice Questions
Explain why a burette is a suitable piece of apparatus for a titration experiment. (2 marks)
Mark 1: The burette allows precise measurement of the volume of titrant (sodium hydroxide) used in the experiment.
Mark 2: The burette can be controlled accurately, delivering the titrant in small, measured volumes, which is necessary for determining the endpoint.
You are asked to design an experiment to determine the concentration of hydrochloric acid using sodium hydroxide solution of known concentration.
(a) Describe how you would select the appropriate apparatus and techniques for this titration experiment.
(b) Explain how you would ensure the accuracy and reliability of your results.
(5 marks)
(a) Selection of Apparatus and Techniques
Mark 1: Choose a burette for titrant delivery, as it allows accurate volume measurements.
Mark 2: Select a pipette for accurate measurement of the volume of hydrochloric acid.
Mark 3: Use a conical flask for the reaction, which allows easy swirling without risk of spillage.
Mark 4: Use a suitable indicator (e.g., phenolphthalein) to determine the endpoint of the titration.
(b) Ensuring Accuracy and Reliability
Mark 1: Calibrate the burette and pipette before use to ensure accurate volume measurements.
Mark 2: Perform a rough titration first to get an approximate endpoint, then conduct more precise titrations.
Mark 3: Repeat the titration several times and calculate the average to improve reliability and reduce random errors.

