OCR Specification focus:
‘Acyl chlorides form esters, acids, and amides; phenol esterifies via acyl chlorides more readily.’
Acyl chlorides are highly reactive derivatives of carboxylic acids that undergo rapid nucleophilic acyl substitution, making them valuable reagents for producing esters, amides, and carboxylic acids efficiently.
Using Acyl Chlorides in Organic Synthesis
Acyl chlorides are among the most reactive members of the carboxylic acid derivative family. Their high reactivity arises from the strongly electron-withdrawing –Cl substituent and the excellent ability of Cl– to act as a leaving group. These features allow a wide range of nucleophiles to attack the carbonyl carbon, enabling predictable and fast transformations that are central to organic synthesis at A-Level.
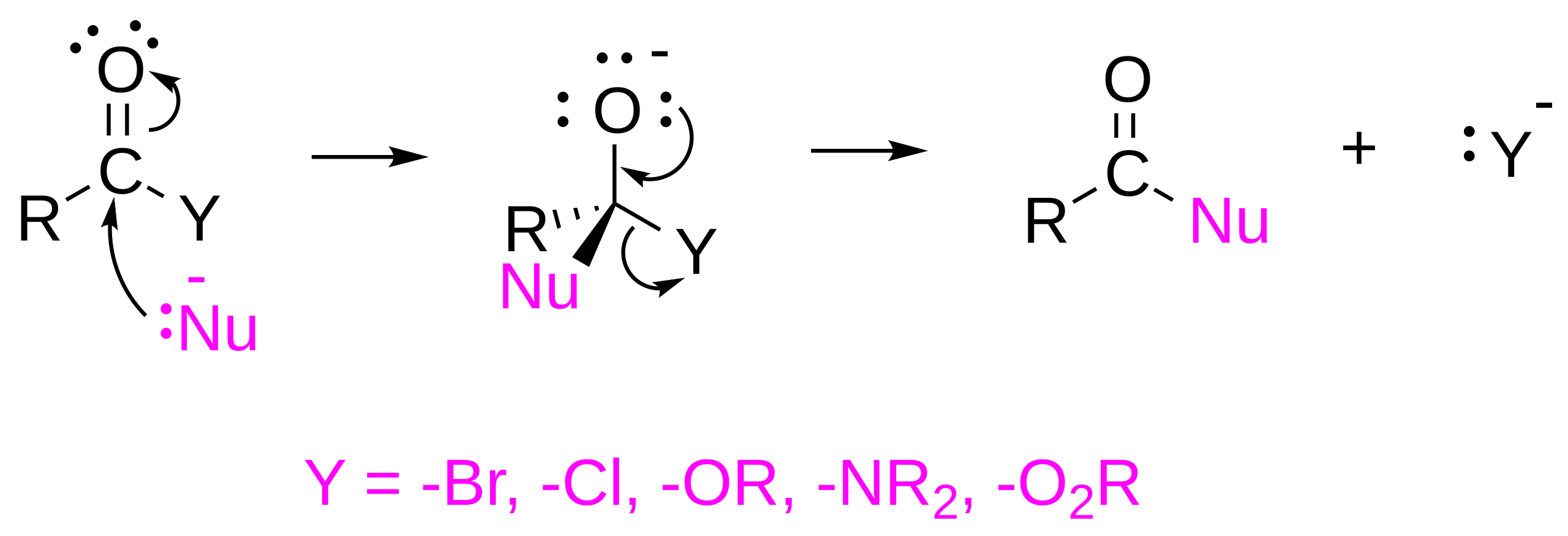
This diagram summarises nucleophilic acyl substitution: a nucleophile attacks the carbonyl carbon to form a tetrahedral intermediate, then the carbonyl reforms as the leaving group is expelled. For acyl chlorides, Cl– is the leaving group, making substitution especially fast. Source
Their reactions follow a common mechanism pattern: nucleophilic attack on the carbonyl carbon, followed by loss of Cl– and formation of the substituted product.
Formation of Esters from Acyl Chlorides
Ester formation using acyl chlorides is a key synthetic route highlighted in the specification. Acyl chlorides react vigorously with alcohols to form esters at room temperature without requiring an acid catalyst.
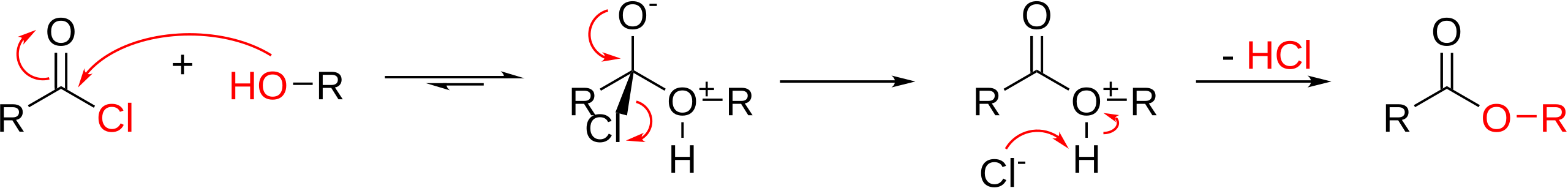
The mechanism shows an alcohol acting as the nucleophile, forming a tetrahedral intermediate before chloride leaves to regenerate the carbonyl. The overall transformation is acyl chloride to ester, with HCl produced. Source
This represents a considerable advantage over traditional esterification with carboxylic acids, which requires concentrated sulfuric acid and reflux conditions.
Alcohols act as nucleophiles, attacking the electrophilic carbonyl carbon of the acyl chloride.
The tetrahedral intermediate collapses, releasing Cl– as the leaving group.
The final product is an ester, and HCl is produced as a by-product.
The reaction is fast and irreversible, which improves yields and simplifies purification. When the nucleophile is phenol, ester formation is significantly more favourable than when phenol reacts with carboxylic acids.
Phenol: An aromatic compound containing a hydroxyl group (–OH) directly bonded to a benzene ring.
Phenol is a weaker nucleophile than aliphatic alcohols, but acyl chlorides are sufficiently reactive to allow phenyl ester formation under mild conditions. This supports the specification point that phenol esterifies more readily via acyl chlorides than via carboxylic acids, which react only slowly with phenol unless strong catalysts are used.
Formation of Carboxylic Acids from Acyl Chlorides
Acyl chlorides react readily with water in a process known as hydrolysis. This produces a carboxylic acid and HCl. Because water is a very strong nucleophile and highly abundant in typical conditions, hydrolysis occurs rapidly, often violently, and must therefore be controlled carefully in practical settings.
Water attacks the carbonyl carbon.
A tetrahedral intermediate forms and collapses.
Cl– is expelled, forming the carboxylic acid.
Hydrolysis often occurs even with moisture in the air, which is why acyl chlorides are usually stored under anhydrous conditions. This reactivity makes acyl chlorides useful for preparing carboxylic acids quickly from intermediates in multi-step synthetic sequences.
Formation of Amides from Acyl Chlorides
Acyl chlorides react with ammonia and amines to form amides, another important transformation in synthetic pathways.
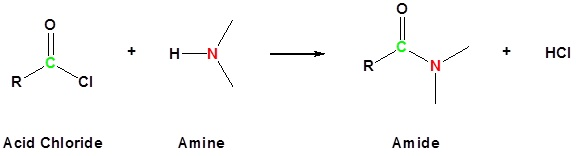
This reaction scheme shows acyl chloride reacting with ammonia or amines to form an amide via nucleophilic substitution. The diagram also indicates HCl formation, explaining why excess amine is often used to neutralise the acid. Source
The reaction is typically rapid and occurs at room temperature. Different amines generate different classes of amides:
NH₃ gives primary amides.
Primary amines give secondary amides.
Secondary amines give tertiary amides.
In all cases, the mechanism follows nucleophilic attack by the amine, formation of the tetrahedral intermediate, and elimination of Cl–.
Because HCl is formed as a by-product, a second equivalent of amine is often added to neutralise the acid. This prevents protonation of the amine nucleophile, maintaining its reactivity.
Amide: An organic compound containing a carbonyl group bonded directly to a nitrogen atom (–CONH₂, –CONHR, or –CONR₂).
This neutralisation step is essential when preparing pure amides and helps avoid side reactions. Acyl chlorides therefore offer a clean, predictable route to amide formation and are frequently used in laboratory synthesis where mild conditions are required.
Mechanistic Overview: Nucleophilic Acyl Substitution
The reactions of acyl chlorides represent classic examples of nucleophilic acyl substitution, a reaction in which a nucleophile replaces the leaving group bonded to the carbonyl carbon. The reactivity of acyl chlorides is significantly greater than that of other carboxylic acid derivatives, such as esters or amides, due to the strong effect of the chlorine substituent.
The carbonyl carbon is made highly δ+ by the electronegative chlorine atom.
Nucleophiles attack easily, forming a tetrahedral intermediate.
The collapse of this intermediate ejects Cl–, forming the substituted product.
This mechanism underpins all transformations involving acyl chlorides and explains why these reagents are so versatile in synthesis.
Factors Affecting Reactivity of Acyl Chlorides
Several structural and environmental factors contribute to their behaviour in organic synthesis:
Electron withdrawal by chlorine increases carbonyl electrophilicity.
Good leaving group ability of chloride facilitates rapid substitution.
Steric effects: bulky acyl chlorides react more slowly because nucleophilic attack is hindered.
Moisture sensitivity means reactions must be conducted under dry conditions unless hydrolysis is desired.
These factors together explain why acyl chlorides serve as key intermediates in designing synthetic pathways for esters, amides, and carboxylic acids, in full alignment with the OCR specification.
FAQ
Acyl chlorides react rapidly with water, even from moisture in the air, producing carboxylic acids and hydrogen chloride gas.
This unwanted hydrolysis reduces yield and can interfere with planned reactions, especially when forming esters or amides.
For this reason, acyl chloride reactions are normally performed using dry glassware and anhydrous solvents to maintain control and maximise product formation.
Hydrogen chloride formed during the reaction can protonate ammonia or amines, converting them into ammonium salts.
This reduces the nucleophilicity of the amine and slows or stops further reaction.
To avoid this, excess amine is often used so that one portion reacts with the acyl chloride while another neutralises the hydrogen chloride.
The chlorine atom strongly withdraws electron density from the carbonyl carbon, increasing its partial positive charge.
Chloride ions are also much better leaving groups than alkoxide or amide ions.
Together, these factors make nucleophilic attack easier and allow reactions to occur rapidly at room temperature without catalysts.
Phenol is a weaker nucleophile than aliphatic alcohols because the lone pair on oxygen is partially delocalised into the aromatic ring.
Carboxylic acids are not reactive enough to compensate for this reduced nucleophilicity.
Acyl chlorides are sufficiently reactive to overcome this limitation, allowing phenyl esters to form quickly under mild conditions.
Their extreme reactivity makes them unstable in aqueous environments.
In biological systems, where water is abundant, acyl chlorides would hydrolyse almost immediately.
As a result, living systems rely on less reactive derivatives, such as esters or amides, to carry out controlled chemical processes.
Practice Questions
An acyl chloride reacts readily with ethanol to form an ester.
State two reasons why this reaction occurs more readily than the reaction between ethanol and a carboxylic acid.
(2 marks)
Award one mark for each correct point.
Acyl chlorides are more reactive because the C=O carbon is more electron-deficient due to the electron-withdrawing chlorine atom. (1 mark)
Chloride ions are good leaving groups, making substitution easier. (1 mark)
Reaction does not require an acid catalyst or heating. (1 mark, accept as an alternative)
The reaction is irreversible, unlike esterification with carboxylic acids. (1 mark, accept as an alternative)
Maximum 2 marks.
An acyl chloride reacts with excess ammonia to form an amide.
a) Name the type of reaction that occurs. (1 mark)
b) Describe the mechanism of this reaction, including the role of ammonia and the formation of the final products. (4 marks)
(5 marks)
a)
Nucleophilic acyl substitution. (1 mark)
b)
Award marks as follows:
Ammonia acts as a nucleophile and attacks the carbonyl carbon of the acyl chloride. (1 mark)
A tetrahedral intermediate is formed. (1 mark)
The intermediate collapses and chloride ions are eliminated as the leaving group. (1 mark)
Hydrogen chloride is formed and is neutralised by excess ammonia to prevent protonation of the nucleophile. (1 mark)
Maximum 4 marks for part b.
Total = 5 marks.

