OCR Specification focus:
'Purify by filtration under reduced pressure, recrystallisation, and measuring melting points.'
Purifying organic solids ensures reliable characterisation and improved yield. This subtopic covers essential purification techniques—filtration under reduced pressure, recrystallisation, and melting-point analysis—used throughout organic synthesis.
Filtration Under Reduced Pressure
Filtration under reduced pressure is a rapid method for isolating a solid product from a reaction mixture or purification solvent. It is essential when collecting crystals formed during recrystallisation because it removes solvent efficiently and produces drier solids than gravity filtration.
Apparatus and Setup
A typical setup includes:
Büchner funnel fitted with filter paper
Büchner flask or side-arm flask
Vacuum source, usually a water pump
Rubber seal to ensure an airtight connection
A slurry of the crude solid is poured onto the funnel while suction draws the liquid through the filter. Maintaining an effective seal prevents pressure leaks and ensures rapid filtration.
Set up a Büchner funnel with filter paper on a side-arm filter flask, ensuring all joints are airtight before applying the vacuum.
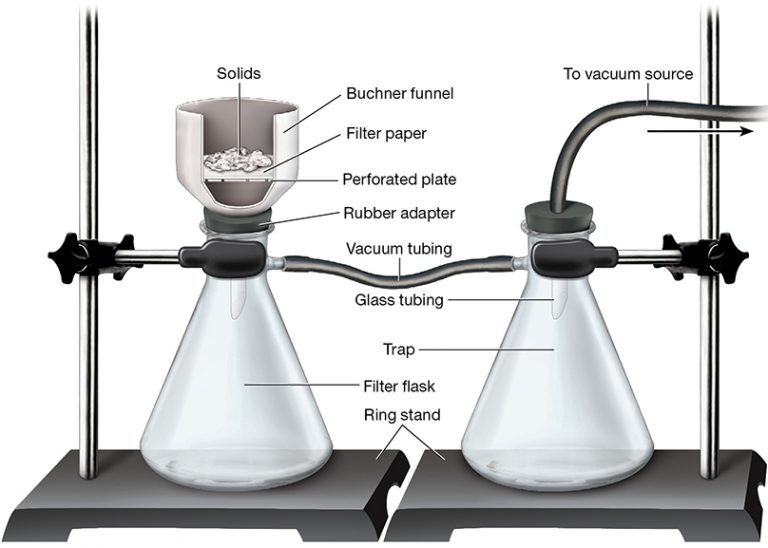
Labelled vacuum filtration setup showing how a Büchner funnel and filter paper sit on a side-arm filter flask connected to a vacuum source. The trap prevents backflow and protects the apparatus during suction. Source
Washing and Drying the Solid
After the main liquid has been removed, the solid is usually washed with a small quantity of cold solvent to remove soluble impurities. Cold solvent minimises the risk of dissolving the desired product.
To improve dryness:
Continue suction for several minutes to remove residual solvent.
Press the solid gently with a spatula to increase contact with air flow.
This technique is particularly helpful when the product is heat-sensitive and cannot be dried thoroughly in an oven.
Recrystallisation
Recrystallisation is a purification method based on differences in solubility between a compound and its impurities. It relies on dissolving the crude solid in a suitable hot solvent and allowing it to crystallise as the solution cools.
Choosing an Appropriate Solvent
A good recrystallisation solvent should:
Dissolve the organic solid when hot
Not dissolve it when cold
Leave impurities either always soluble or always insoluble
Be volatile enough to allow easy removal after filtration
Common solvents include ethanol, water, ethyl ethanoate, and propanone. Selection depends on balancing solubility and practicality for heating and cooling.
Dissolving the Crude Product
The crude solid is heated gently with a minimum volume of hot solvent. Using excess solvent reduces crystal yield, so the smallest quantity necessary should be used. If insoluble impurities remain, the hot mixture is hot-filtered through fluted paper to remove them before crystallisation occurs.
Recrystallisation purifies an impure solid by dissolving it in the minimum volume of hot solvent, then allowing it to cool so purer crystals form.
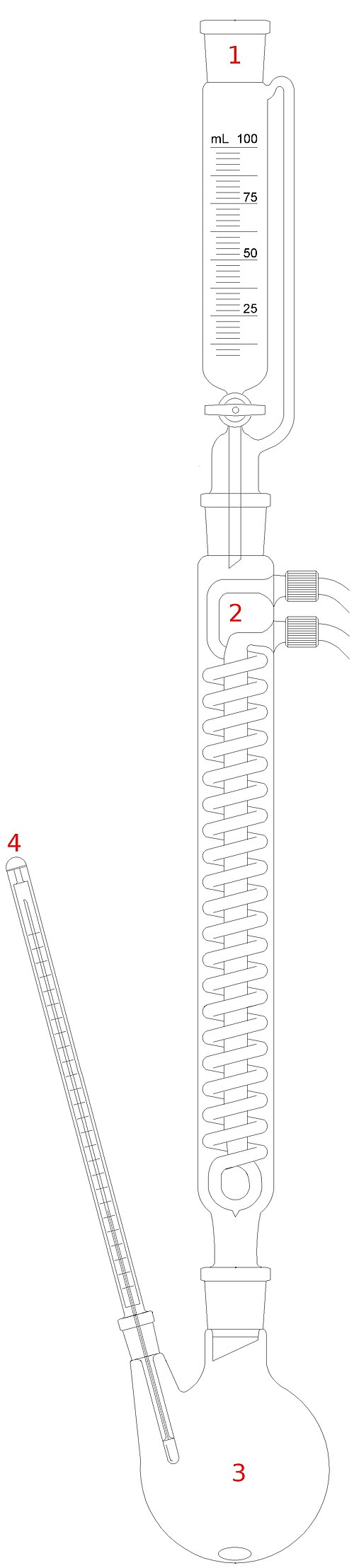
Diagram of a recrystallisation apparatus showing heated glassware and a condenser to reduce solvent loss. The inclusion of a dropping funnel and thermometer adds detail beyond OCR requirements but reflects some laboratory setups. Source
Crystal Formation
As the hot saturated solution cools:
Solubility decreases
Pure product molecules arrange into an ordered lattice
Impurities remain dissolved or precipitate separately
Cooling stages often include:
Initial bench cooling, allowing crystal nuclei to form slowly
Ice-bath cooling, promoting further crystallisation
Slower crystal growth usually gives purer crystals because impurities have less tendency to become trapped in the lattice.
Collecting Crystals
Once crystallisation is complete, the crystals are isolated by filtration under reduced pressure. They may then be washed with a small amount of cold solvent to remove traces of mother liquor. Effective washing prevents impurities from adhering to the crystal surfaces.
Melting-Point Determination
Measuring melting points is a key method for assessing purity. Pure organic solids have a sharp melting point, usually within a range of 1–2 °C. Impure solids melt over a wider temperature range and at a lower temperature.
Using a Melting-Point Apparatus
Organic solids are tested using a capillary tube in one of two common devices:
Thiele tube heated with an oil bath
Digital melting-point machine
A small amount of dried solid is packed into a capillary tube. The temperature is raised gradually to observe the point at which the first and last particles melt.
Melting-point range: The temperature interval between the first sign of melting and the point at which the solid becomes fully liquid.
A sharp melting-point range indicates high purity because a uniform crystal lattice breaks down at a consistent temperature.
Before using melting-point data confidently, the thermometer or temperature probe must be calibrated using substances of known melting point.
A pure organic solid shows a sharp melting point, whereas impurities typically lower the melting point and broaden the melting range.
Photograph of an electronic melting point apparatus used to measure the temperature range over which a solid melts. The digital features exceed basic school equipment but illustrate the same purity-testing principle. Source
Integrating the Techniques in Organic Synthesis
In typical organic preparations, purification follows a consistent sequence:
Isolate the crude solid using filtration under reduced pressure
Dissolve and recrystallise the solid to remove impurities
Collect and dry the purified crystals
Verify purity using melting-point analysis
Each technique reinforces the next, ensuring the final product is suitable for further reactions or structural identification.
Key Practical Considerations
When applying these techniques, chemists evaluate several factors:
Solvent choice strongly affects recrystallisation efficiency
Rate of cooling influences crystal purity and size
Thorough drying is essential before melting-point analysis
Equipment cleanliness prevents contamination
Use of minimal solvent maximises yield
These considerations ensure reproducible results and support accurate characterisation across organic chemistry experiments.
FAQ
Using the minimum volume ensures the product is just fully dissolved when hot. Excess solvent keeps more product dissolved when cooled, reducing yield.
A smaller volume also encourages efficient crystal formation, as the solution becomes saturated more easily during cooling.
Cold solvent removes surface impurities without dissolving significant amounts of the desired product.
Warm solvent would increase solubility and can dissolve the crystals themselves, lowering the final yield.
Oiling out occurs when a substance separates as a liquid instead of forming crystals, often due to poor solvent choice or rapid cooling.
It can be reduced by:
Reheating and adding a small amount of fresh solvent
Allowing slower cooling
Scratching the glass to encourage crystal nucleation
Residual solvent behaves as an impurity, lowering and broadening the melting point range.
Dry samples ensure the measured melting point reflects only the solid’s purity rather than solvent contamination.
Melting occurs gradually as different parts of the crystal lattice break down.
Recording a range captures the first and last signs of melting, allowing more accurate assessment of purity than a single temperature reading.
Practice Questions
A student prepares an organic solid by recrystallisation.
State two reasons why filtration under reduced pressure is used instead of gravity filtration when collecting the crystals.
(2 marks)
Award one mark for each correct point, up to a maximum of two marks.
Filtration under reduced pressure is faster than gravity filtration.
It produces a drier solid by removing more solvent.
It is suitable for collecting crystals formed during recrystallisation.
A crude organic solid is purified by recrystallisation using ethanol as the solvent.
(a) Describe how the student would carry out the recrystallisation to obtain a pure solid. (4 marks)
(b) Explain how measuring the melting point of the product can be used to assess its purity. (1 mark)
(5 marks)
(a) Recrystallisation method (4 marks)
Award one mark for each correct step, up to a maximum of four marks.
Dissolve the crude solid in the minimum volume of hot ethanol.
Filter the hot solution to remove insoluble impurities.
Allow the solution to cool slowly so crystals form.
Collect the crystals by filtration under reduced pressure (or wash with cold solvent).
(b) Melting point and purity (1 mark)
Award one mark for a correct explanation.
A pure solid has a sharp melting point (or narrow melting range), whereas impurities lower and broaden the melting point range.

