OCR Specification focus:
‘Identify functional groups in multi-functional molecules and predict their properties and reactions.’
Organic molecules often contain several functional groups, and recognising these groups allows chemists to predict characteristic reactions, physical behaviour, and reactivity patterns. This subtopic focuses on reliably identifying these groups in multi-functional molecules and understanding how their presence influences chemical behaviour.
Understanding Functional Groups
Functional groups are specific atoms or groups of atoms that dictate the chemistry of organic molecules. They determine how a compound reacts, its polarity, solubility, and intermolecular forces.
Functional Group: A specific atom or group of atoms within a molecule responsible for its characteristic chemical reactions.
When analysing an unfamiliar structure, recognising these groups is the first step in deducing how the molecule will behave in a range of conditions.
Common Functional Groups Relevant to OCR A-Level Chemistry
Students must confidently identify a range of groups that appear throughout the course:
Alkanes – saturated hydrocarbons containing only C–C and C–H single bonds.
Alkenes – molecules containing a C=C double bond.
Alcohols – compounds with a hydroxyl (–OH) group.
Aldehydes – possess a carbonyl group at the end of a carbon chain.
Ketones – contain a carbonyl group within the carbon chain.
Carboxylic acids – include a carboxyl (–COOH) group.
Esters – have the linkage –COO– between alkyl or aryl groups.
Amines – contain an –NH2, –NHR or –NR2 group.
Amides – contain the –CONH2, –CONHR or –CONR2 group.
Nitriles – include the –C≡N group.
Haloalkanes – feature halogen atoms bonded to carbon chains.
These groups appear in multi-functional structures and their interactions influence the chemistry of the molecule as a whole.
Identifying Functional Groups in Multi-Functional Molecules
A molecule containing several functional groups will display combined or competing influences on reactivity. Students should learn to:
Scan the structure systematically, identifying key bond types and heteroatoms.
Recognise patterns such as carbonyl groups, nitrogen-containing groups, and oxygen-based substituents.
Consider how proximity of groups may modify typical reactivity.
Structural Clues for Identification
To accurately identify functional groups, observe:
Bond types: C=C, C≡N, C=O, and O–H are strong indicators of group type.
Heteroatoms: Nitrogen, oxygen, and halogens often mark specific groups.
Group arrangement: Whether the carbonyl is terminal (aldehyde) or internal (ketone), or whether nitrogen is attached directly to a carbonyl (amide).
These clues help determine not only the group present but also how it shapes the molecule’s chemistry.
A particularly important set of groups to recognise are amines, amides, and nitriles, because nitrogen lone pairs often control basicity and nucleophilic behaviour.
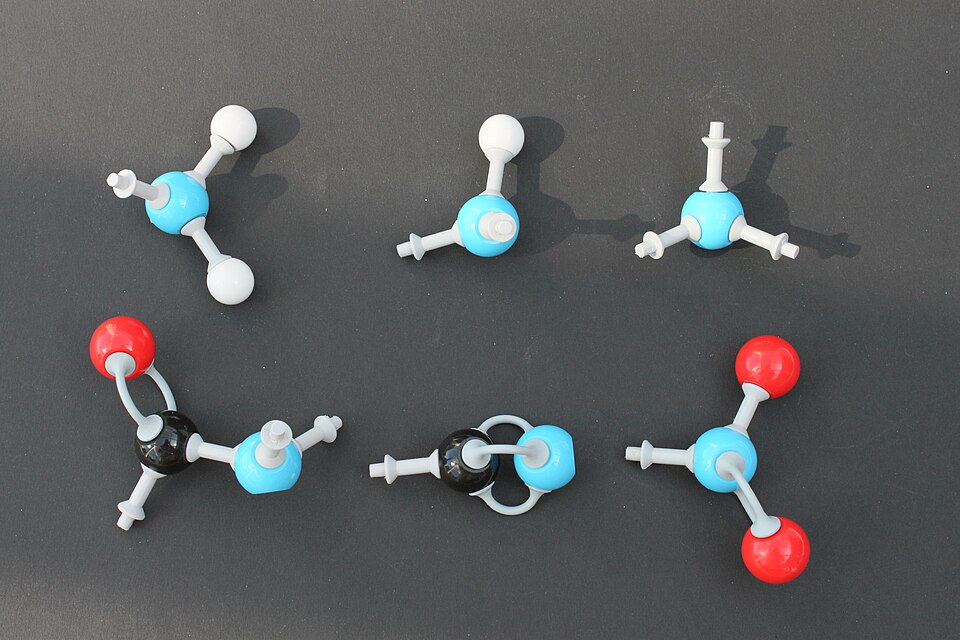
This diagram shows common nitrogen-containing functional groups including amines, amides and nitriles. The bonding around nitrogen helps distinguish basic amines from less basic amides and linear nitriles. A nitro group is also shown, which is useful for comparison but goes beyond the core syllabus emphasis. Source
Predicting Properties from Functional Groups
Functional groups significantly affect the physical properties of organic molecules.
Polarity and Intermolecular Forces
Functional groups influence molecular polarity, which in turn affects boiling point, solubility, and volatility.
Alcohols, carboxylic acids, and amides form hydrogen bonds, giving high boiling points.
Aldehydes and ketones exhibit dipole–dipole interactions because of the polar carbonyl group.
Alkanes and alkenes show only London dispersion forces and have lower boiling points.
Polarity: Uneven distribution of electron density leading to partial positive and negative charges within a molecule.
Intermolecular forces should be assessed to predict physical properties, particularly trends in solubility and volatility.
Compounds with O–H or N–H bonds can form hydrogen bonds, which usually increases boiling point and often increases water solubility compared with molecules of similar size.
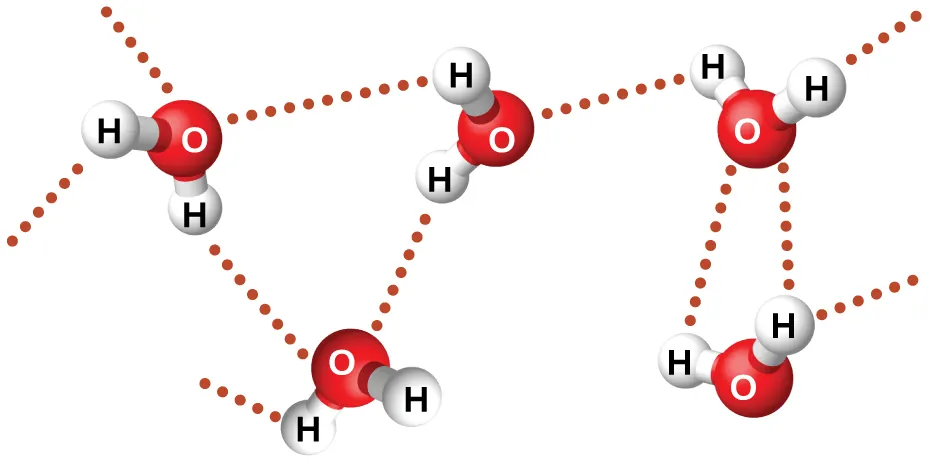
The dotted lines represent hydrogen bonds between neighbouring molecules. Similar interactions occur between organic molecules containing O–H or N–H groups. Hydrogen bonding explains higher boiling points and greater water solubility for many oxygen- and nitrogen-containing compounds. Source
Solubility
Functional groups containing heteroatoms capable of hydrogen bonding typically enhance solubility in water:
Alcohols with short chains dissolve readily.
Carboxylic acids form hydrogen bonds with water and may ionise in alkaline conditions.
Amines act as bases and become protonated, increasing solubility in acidic environments.
Larger non-polar regions decrease solubility even when polar groups are present.
A mix of polar and non-polar groups leads to molecules with intermediate solubility characteristics.
Predicting Chemical Reactivity
Functional groups determine how a molecule reacts with common reagents.
Nucleophiles, Electrophiles, and Characteristic Reactions
Reactive centres form where electron density is high or low:
Carbonyl carbons (in aldehydes, ketones, carboxylic acids, esters, and amides) act as electrophilic centres.
Nitrogen-containing groups such as amines act as nucleophiles due to their lone pairs.
Alkenes react with electrophiles via addition to the C=C bond.
Electrophile: A species that accepts an electron pair to form a new covalent bond.
The presence of multiple groups influences reaction pathways. For instance, an amino acid contains both an amine and a carboxylic acid group, allowing it to undergo acid–base reactions and condensation reactions depending on conditions.
Reactivity in Multi-Functional Molecules
Reactivity is influenced by both the group itself and its environment within the molecule:
Electron-withdrawing groups near nucleophilic sites decrease nucleophilicity.
Electron-donating groups near electrophilic centres reduce their reactivity.
Groups may undergo intramolecular reactions if positioned favourably, such as ester or amide formation.
Proximity can enhance or restrict access to reactive centres.
These considerations help predict how a molecule participates in reactions such as esterification, nucleophilic addition, or hydrolysis.
Functional Group Compatibility and Reaction Conditions
Different groups require specific reagents or conditions:
Acid-sensitive groups may be destroyed under strongly acidic conditions.
Bases may deprotonate acidic groups such as phenols or carboxylic acids.
High temperatures can cause unwanted side reactions, particularly in molecules with multiple reactive sites.
Understanding the stability of each group helps in predicting feasible reaction conditions for synthesis and analysis.
Oxygen-containing groups such as alcohols, aldehydes, ketones, carboxylic acids, esters, and ethers are often the quickest way to predict polarity, boiling point, and likely reaction pathways.
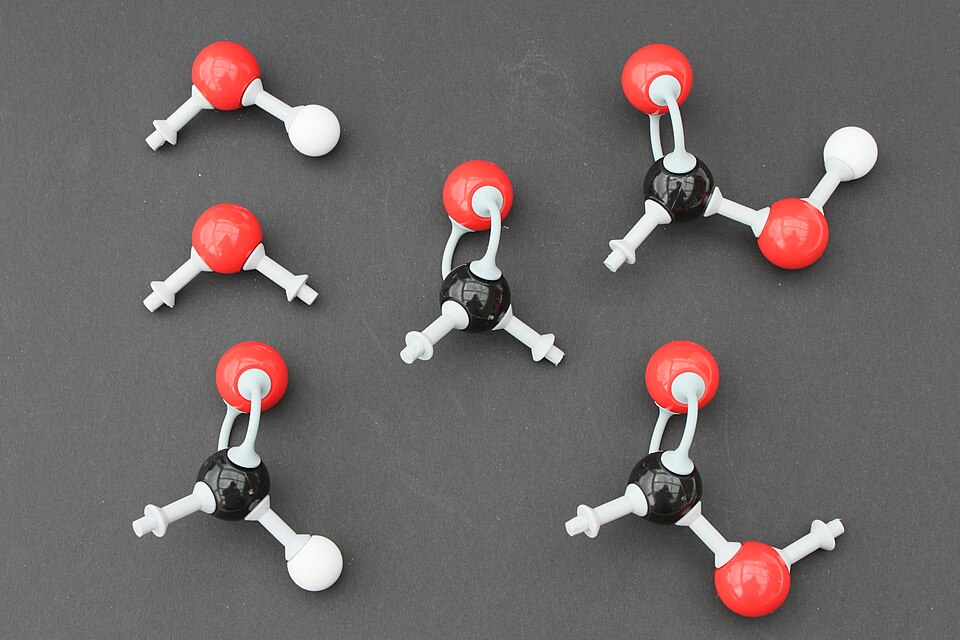
This image compares the main oxygen-containing functional groups encountered at A-level. The presence and position of the carbonyl group are key to distinguishing aldehydes, ketones, acids and esters. These structural differences explain contrasting physical properties and reactivity. Source
Summary of Skills for OCR A-Level Chemistry
To meet the specification requirement, students must be able to:
Identify functional groups in molecules containing multiple reactive sites.
Predict their physical properties based on polarity and intermolecular forces.
Anticipate chemical reactions that each group is likely to undergo.
The ability to read molecular structures confidently and interpret their reactive behaviour underpins all higher-level organic synthesis and analysis.
FAQ
Functional groups influence pH through acid–base behaviour in water.
Carboxylic acid groups can donate protons, lowering pH, while amine groups can accept protons, raising pH. In molecules containing both groups, such as amino-acid-like structures, the effects can partially cancel, leading to near-neutral solutions.
The overall pH depends on:
The relative strengths of the acidic and basic groups
Whether the groups are protonated or deprotonated under the conditions
Differences in boiling point arise from the type of intermolecular forces present.
Molecules with functional groups capable of hydrogen bonding, such as alcohols or amides, require more energy to separate. Non-polar molecules of similar size rely only on London dispersion forces, which are weaker.
Even a single functional group change can significantly alter boiling point.
The location of a functional group influences accessibility and electronic effects.
Groups near bulky alkyl chains may be sterically hindered, reducing reaction rate. Functional groups close to electron-withdrawing or electron-donating groups can have altered reactivity due to changes in electron density.
This is particularly important in multi-functional molecules where groups interact.
In amides, the nitrogen lone pair is delocalised into the adjacent carbonyl group.
This reduces the availability of the lone pair to accept protons, making amides very weak bases. In amines, the lone pair is localised and readily available, so they act as stronger bases.
This difference explains contrasting chemical behaviour despite similar atoms.
Solvent choice depends on polarity and intermolecular interactions.
Polar functional groups favour polar solvents due to dipole–dipole interactions or hydrogen bonding. Non-polar molecules dissolve better in non-polar solvents.
In reactions, the solvent must dissolve reactants without reacting with sensitive functional groups, ensuring the intended reaction pathway occurs.
Practice Questions
The molecule below contains more than one functional group.
State two different functional groups present in an organic molecule that contains both the –NH2 group and the –COOH group, and name one property that can be predicted from these functional groups.
(2 marks)
Identifies a correct functional group: amine OR carboxylic acid (1 mark)
States a valid predicted property linked to functional groups, e.g. ability to act as a base, acidic behaviour, hydrogen bonding, high boiling point, or solubility in water (1 mark)
Maximum 2 marks
An organic compound has the structural formula CH3CH(OH)CONH2.
a) Identify all the functional groups present in this molecule.
b) Using these functional groups, predict and explain two physical or chemical properties of the compound.
(5 marks)
a) Identification of functional groups (3 marks total)
Alcohol (–OH) correctly identified (1 mark)
Amide (–CONH2) correctly identified (1 mark)
Carbonyl group within the amide correctly recognised OR amide clearly distinguished from an amine (1 mark)
b) Properties and explanation (2 marks total)
One correct property predicted, e.g. hydrogen bonding, high boiling point, solubility in water, or limited basicity (1 mark)
Explanation linked to functional groups, e.g. presence of O–H or N–H bonds allowing hydrogen bonding, polar C=O group causing dipole–dipole interactions (1 mark)
Maximum 5 marks

