OCR Specification focus:
‘Use Quickfit apparatus with distillation and heating under reflux for organic preparations.’
Organic preparation requires careful use of specialised laboratory equipment to ensure reactions proceed safely, efficiently, and with minimal loss of product. This section outlines essential techniques.
Organic Preparation Techniques
Organic synthesis often involves controlling reaction conditions, assembling appropriate apparatus, and selecting suitable purification steps. OCR expects students to understand how Quickfit glassware, distillation, and heating under reflux are used to prepare organic compounds safely and effectively.
Quickfit Apparatus
Quickfit is a modular system of borosilicate glassware designed to connect via standard taper joints, providing airtight assemblies suitable for heating and solvent use.
Quickfit apparatus: A set of interchangeable glass components with standard ground-glass joints used to build leak-proof systems for organic preparations.
Quickfit assemblies are favoured because they resist thermal shock, form tight seals, and allow versatile configurations. Students should be able to recognise commonly used pieces:
Round-bottom flasks — for heating reaction mixtures uniformly.
Condensers — e.g., Liebig, Allihn; chosen based on cooling efficiency required.
Still heads, distillation adapters, and thermometer pockets — used when controlling boiling points in distillation setups.
Dropping funnels — for adding reagents slowly in controlled conditions.
Claissen adapters — to allow simultaneous insertion of a thermometer and condenser.
Quickfit assemblies rely on standard taper ground-glass joints, secured with Keck clips, and supported by clamps to prevent strain on the glass.

This photograph shows a socket-and-cone ground-glass joint secured with Keck clips, demonstrating how Quickfit glassware components are safely connected to prevent separation during heating. Although shown on a rotary evaporator, the joint mechanism is identical to that used in reflux and distillation assemblies. Source
Heating Under Reflux
Heating under reflux is an essential method for maintaining a reaction mixture at its boiling point without losing volatile components. This prevents solvent evaporation while enabling reactions requiring prolonged heating.
Reflux: A technique in which vapours produced by heating a reaction mixture condense and return to the flask, allowing continuous boiling without loss of solvent.
A typical reflux setup includes:
A round-bottom flask containing the reaction mixture.
A vertical condenser, water-cooled, placed above the flask.
A heat source, commonly a heating mantle or water bath.
Anti-bumping granules to promote smooth boiling.
During heating under reflux, vapour rises, condenses in the water-cooled condenser, and returns to the flask so the solvent is not lost.
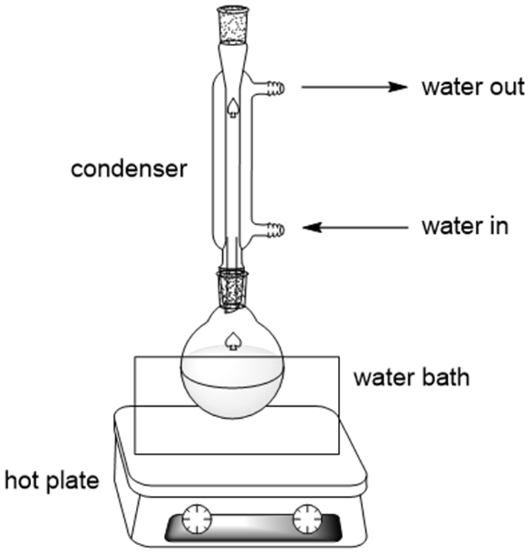
This diagram illustrates a standard reflux apparatus with a vertical water-cooled condenser allowing vapour to condense and return to the reaction flask. Water enters at the bottom and exits at the top to maximise cooling efficiency. The heat source may vary depending on solvent boiling point. Source
A reflux system must never be sealed, as pressure build-up can cause glassware to shatter. The condenser must remain upright and water must flow from the bottom to the top to maximise cooling efficiency.
During reflux:
Reaction progress is often monitored by thin-layer chromatography (TLC).
The heating period depends on reaction kinetics and solvent boiling point.
Safety considerations include ensuring secure clamp support and removing flammable materials from the heat source.
Distillation
Distillation is used either to purify a product or to separate substances based on differences in boiling point.
Distillation: A separation technique in which a liquid is vaporised, condensed, and collected to isolate components with different boiling points.
Students must understand when distillation is applied during organic preparations:
To isolate a product formed by a reaction (e.g., esterification).
To remove a low-boiling impurity.
To dry a solvent by removing water.
To purify a crude liquid product after reflux.
A simple distillation setup includes:
Round-bottom flask with the mixture.
Still head leading to a condenser.
Thermometer to monitor vapour temperature.
Receiver to collect distillate.
Distillation is used to separate and collect a volatile liquid by vaporisation followed by condensation in a water-cooled condenser.
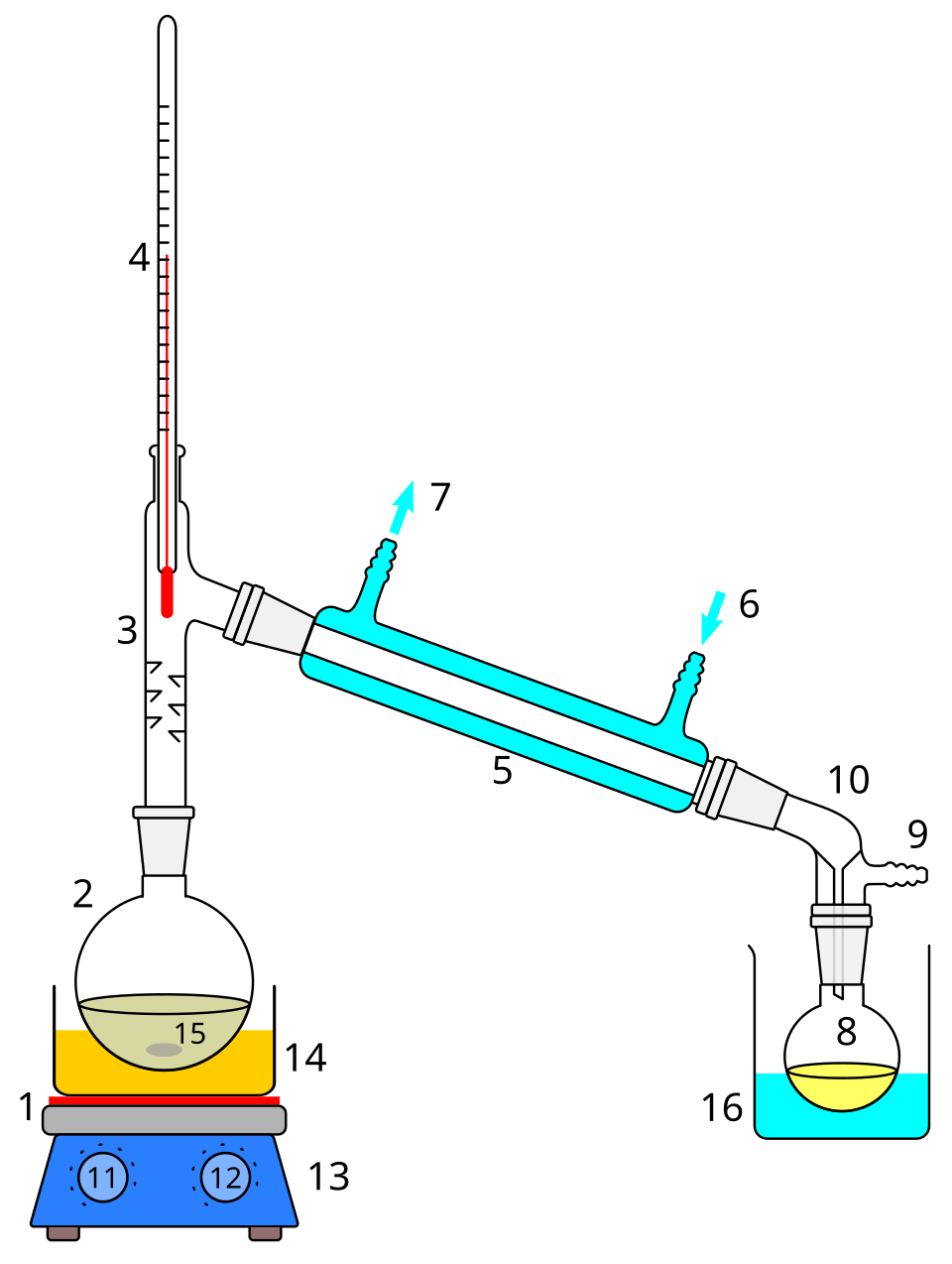
This schematic shows a simple distillation apparatus, including a heated round-bottom flask, thermometer in the still head, Liebig condenser, and receiving flask. Vapour condenses in the condenser and is collected as distillate. Some versions include additional joints not required for OCR A-Level study. Source
Fractional distillation is used when components have closer boiling points. A fractionating column improves vapour–liquid contact, increasing separation efficiency.
Between distillation and reflux lies a key procedural difference:
Reflux keeps volatile components in the flask.
Distillation removes them deliberately.
Practical Steps in Organic Preparations
Organic preparations typically follow a consistent sequence. OCR expects students to know this general workflow:
1. Assembling Apparatus
Select appropriate Quickfit components.
Inspect for cracks and ensure joints are clean.
Use grease sparingly to improve joint seals where necessary.
Clamp securely but avoid overtightening, which stresses the glass.
2. Charging the Reaction Flask
Add reactants and solvent carefully.
Introduce anti-bumping granules before heating.
Use a funnel to avoid spillage of corrosive liquids.
3. Choosing the Heating Method
Heating mantle — best for flammable solvents.
Water bath — for gentle heating.
Oil bath — for high-temperature stability.
Direct flame should generally be avoided unless using non-flammable solvents.
4. Monitoring the Reaction
Maintain steady boiling during reflux.
Observe changes such as colour, precipitation, or smell (fume cupboard required).
Use TLC or sampling where appropriate.
5. Post-Reaction Operations
Cool the mixture before dismantling apparatus.
If required, distil immediately to isolate the product or remove unwanted volatile materials.
Transfer mixtures using wash bottles and glass funnels, ensuring minimal loss.
Safety Considerations
Organic preparation must adhere to strict safety practices:
Always work in a fume cupboard when handling volatile or toxic reagents.
Wear heat-resistant gloves when dismantling hot glassware.
Ensure all joints are supported by clamps to prevent tipping.
Keep water hoses secure to prevent flooding.
Common Errors to Avoid
Sealing a reflux system — risk of explosion.
Incorrect water flow direction in a condenser — reduces cooling efficiency.
Overfilling the reaction flask — causes bumping and poor mixing.
Using an open flame with flammable solvents — extreme fire hazard.
Key Equipment Checklist
Students should recognise and be able to label:
Round-bottom flask
Condenser (Liebig or Allihn)
Still head
Fractionating column
Dropping funnel
Receiver adapter
Heating mantle or water bath
These elements form the basis of safe and reliable organic preparation using the Quickfit system, as required by the OCR specification.
FAQ
Anti-bumping granules provide nucleation sites that allow bubbles of vapour to form smoothly.
This prevents sudden, violent boiling known as bumping, which can cause loss of reaction mixture or damage to glassware. Granules must be added before heating begins, as adding them to a hot liquid can cause rapid boiling.
Many organic solvents are volatile and flammable, making open flames hazardous.
A heating mantle provides:
Even heating around the round-bottom flask
Reduced fire risk compared to naked flames
Better temperature control for prolonged heating
This makes heating mantles safer and more suitable for reflux and distillation.
Water entering from the bottom ensures the condenser jacket fills completely.
This creates efficient cooling along the entire length of the condenser, preventing vapour from escaping uncondensed. Top-to-bottom flow can leave air pockets, reducing cooling efficiency and increasing solvent loss.
Round-bottom flasks distribute heat evenly across their curved surface.
This reduces localised overheating and minimises the risk of cracking. They also fit securely with Quickfit joints, allowing leak-proof assembly of reflux and distillation apparatus.
Hot glassware can cause burns and may crack if handled or exposed to cold surfaces.
Cooling allows pressure inside the apparatus to equalise and reduces the risk of joint seizure. This ensures safer dismantling and protects both the experimenter and the equipment.
Practice Questions
Explain why heating under reflux is used during many organic preparations.
(2 marks)
Award marks as follows:
One mark for stating that heating under reflux allows the reaction mixture to be heated at its boiling point.
One mark for stating that reflux prevents loss of volatile reactants or solvent by condensation and return to the flask.
A student prepares an organic liquid by heating a reaction mixture under reflux and then purifying the product by simple distillation.
Describe how the apparatus and procedures used in heating under reflux differ from those used in simple distillation.
You should refer to the purpose of each technique and the key features of the apparatus.
(5 marks)
Award marks as follows:
One mark for stating that heating under reflux is used to allow a reaction to proceed for an extended time without loss of solvent.
One mark for describing the use of a vertical condenser in reflux, with vapour condensing and returning to the reaction flask.
One mark for stating that simple distillation is used to separate or purify a liquid product by removing it from the reaction mixture.
One mark for describing that in distillation, vapour passes through a still head and condenser and is collected in a receiver.
One mark for clearly contrasting the outcomes, e.g. reflux keeps volatile substances in the flask, whereas distillation collects the condensed liquid as the product.

