OCR Specification focus:
‘Appreciate how experiments led to acceptance of the photon model in physics.’
Early twentieth-century physics transformed our understanding of light and matter. A series of experiments revealed that electromagnetic radiation behaves as discrete photons, revolutionising classical wave theories and forming the foundation of quantum physics.
From Classical Wave Theory to Quantum Concepts
By the late 1800s, light was well understood as a wave phenomenon, supported by experiments such as Young’s double-slit interference and diffraction patterns. The electromagnetic wave model, proposed by James Clerk Maxwell, unified light with electricity and magnetism, describing it as oscillating electric and magnetic fields travelling through space.
However, this wave theory could not explain several puzzling observations in radiation and atomic behaviour. These anomalies prompted a series of investigations that ultimately led to the photon model — the concept that electromagnetic radiation consists of discrete energy packets.
Blackbody Radiation and the Ultraviolet Catastrophe
The Problem of Blackbody Radiation
A blackbody is an idealised object that absorbs and emits all frequencies of radiation. Classical physics, through the Rayleigh–Jeans law, predicted that as wavelength decreased, emitted energy would increase without bound — the so-called ultraviolet catastrophe. This was clearly contradicted by experimental evidence, which showed that radiation intensity peaked at a certain wavelength and then decreased.
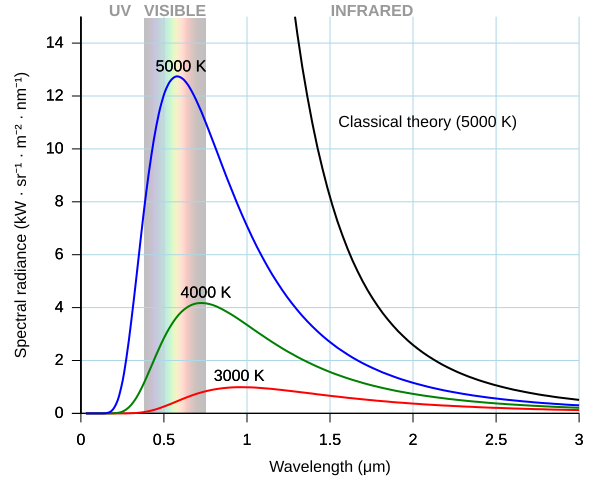
Comparison of blackbody radiation predicted by Planck’s law with the classical Rayleigh–Jeans approximation. The classical curve diverges at short wavelengths (the ultraviolet catastrophe), whereas Planck’s quantised model matches observation. The dashed curves for Wien and Rayleigh–Jeans are extra context beyond the OCR syllabus but help show why quantisation was needed. Source.
Planck’s Quantisation Hypothesis
In 1900, Max Planck resolved this paradox by proposing that electromagnetic energy could only be emitted or absorbed in discrete amounts, or quanta.
Quantum: The smallest discrete amount of a physical property that can exist, such as energy in electromagnetic radiation.
He expressed this idea mathematically as:
EQUATION
—-----------------------------------------------------------------
Energy of a Photon (E) = h × f
E = Energy of the photon (J)
h = Planck’s constant (6.63 × 10⁻³⁴ J·s)
f = Frequency of radiation (Hz)
—-----------------------------------------------------------------
This bold assumption matched observed data perfectly and introduced Planck’s constant, a fundamental quantity linking energy and frequency. It marked the first appearance of quantisation in physics, challenging the continuous energy distributions predicted by classical theory.
Einstein and the Photoelectric Effect
Experimental Evidence for Photons
In 1905, Albert Einstein expanded Planck’s concept, applying it to light itself. He proposed that light was made up of individual photons, each carrying energy E = hf.
The photoelectric effect — the emission of electrons from a metal surface when illuminated by light — provided striking evidence for this particle-like behaviour. Classical wave theory predicted that:
Increasing light intensity should increase the kinetic energy of emitted electrons.
Any frequency of light, given sufficient time, should eventually eject electrons.
However, experiments demonstrated that:
Electrons were emitted only if the light frequency exceeded a threshold frequency.
Increasing light intensity only affected the rate of emission, not the energy of individual electrons.
These results could only be explained if light interacted with electrons as discrete photons, each transferring a quantised packet of energy.
Einstein’s Photon Model
Einstein reasoned that a single photon collides with a single electron, transferring its energy according to:
EQUATION
—-----------------------------------------------------------------
Einstein’s Photoelectric Equation: hf = Φ + KEₘₐₓ
hf = Energy of incoming photon (J)
Φ = Work function of the material (J)
KEₘₐₓ = Maximum kinetic energy of emitted electrons (J)
—-----------------------------------------------------------------
This relationship showed that photon energy must exceed the work function for emission to occur, firmly supporting the particulate nature of light.
Supporting Experimental Developments
Millikan’s Verification
Physicist Robert Millikan, initially sceptical of Einstein’s theory, conducted detailed experiments measuring the stopping potential for different light frequencies. His results not only verified Einstein’s photoelectric equation but also provided an independent measurement of Planck’s constant consistent with Planck’s blackbody radiation results.
This remarkable agreement strengthened the photon model’s credibility and integrated the concept of quantisation across different physical phenomena.
LED and Semiconductor Evidence
Modern demonstrations, such as using light-emitting diodes (LEDs) to estimate Planck’s constant, further illustrate photon quantisation. LEDs emit light when electrons recombine with holes, releasing discrete photons of energy corresponding to E = hf. Measuring the threshold voltage for LEDs of different colours enables students to estimate h, linking twentieth-century theory to accessible classroom experiments.
The Shift to Quantum Theory
The Dual Nature of Light
By the 1920s, additional experiments revealed that particles like electrons also exhibited wave-like properties, leading to the principle of wave–particle duality. This duality confirmed that both light and matter could behave as waves or particles depending on the experiment, solidifying the quantum interpretation.
Impact on Modern Physics
The acceptance of the photon model marked a turning point in scientific thought. It introduced a probabilistic and quantised view of nature, paving the way for quantum mechanics, atomic models, and technologies such as lasers, photodiodes, and solar cells.
Key experiments in this intellectual evolution included:
Planck’s blackbody radiation (1900): quantisation of energy emission.
Einstein’s photoelectric explanation (1905): introduction of photons.
Millikan’s verification (1916): empirical support for quantisation.
Compton scattering (1923): photon momentum transfer confirmed.
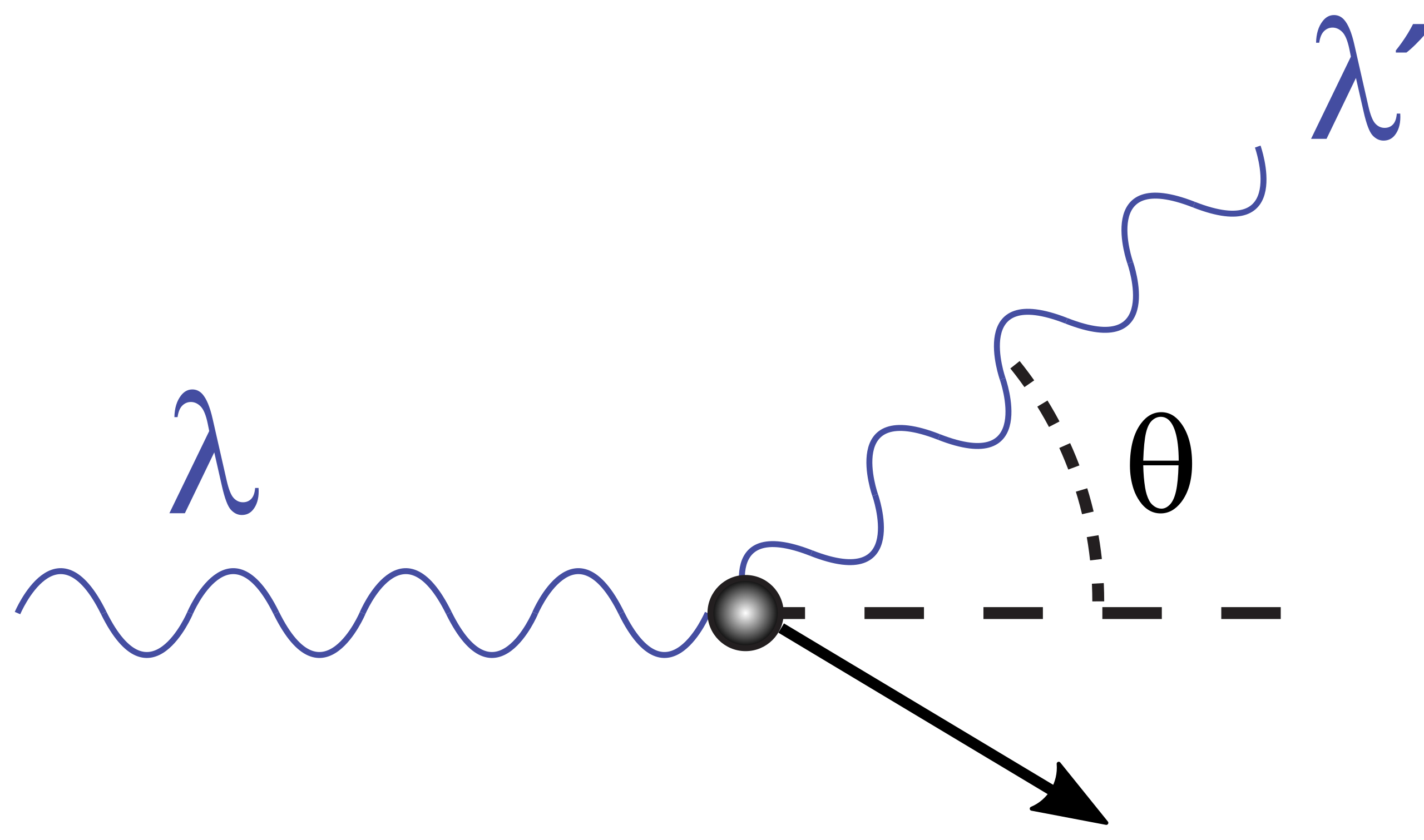
Schematic of Compton scattering: an incident photon strikes an electron, and a scattered photon emerges at angle θ\thetaθ with longer wavelength (lower energy). This momentum-transfer picture is a decisive particle-like interaction of light. The diagram is intentionally minimal and includes no algebra beyond the syllabus. Source.
Each experiment progressively dismantled classical continuous models, replacing them with a discrete, probabilistic framework that remains central to modern physics.
Significance of Experimental Evidence
The acceptance of the photon model was not immediate; it developed through rigorous experimental confirmation. This reflects the scientific process — ideas are tested, refined, and accepted based on empirical success.
The development of quantum ideas thus exemplifies how experimental results drive theoretical change, shifting physics from deterministic classical models to a quantised, probabilistic understanding of the microscopic world.
FAQ
Before Planck, energy was believed to vary continuously. Planck’s suggestion that energy could only be emitted or absorbed in discrete packets (quanta) directly contradicted classical physics.
This was revolutionary because it introduced a statistical and probabilistic view of energy emission rather than a continuous one. It marked the first step towards quantum theory, showing that microscopic processes did not always obey classical rules.
Millikan measured the stopping potential of photoelectrons for various light frequencies.
He found a straight-line relationship between the maximum kinetic energy of emitted electrons and the light’s frequency, with the slope corresponding to Planck’s constant. This matched Einstein’s photoelectric equation precisely.
Although Millikan expected to disprove Einstein, his data provided one of the strongest confirmations of the photon model of light.
Compton scattering demonstrated that photons carry momentum as well as energy.
When X-rays struck electrons in a material, the scattered radiation showed an increase in wavelength, consistent with a collision between a photon and an electron.
This proved that photons behave like particles during interactions, further validating the photon concept and showing that electromagnetic radiation has both wave and particle characteristics.
Once photons were recognised as particles with quantised energy, scientists began applying similar ideas to matter itself.
This led to de Broglie’s hypothesis that particles such as electrons also have wave-like properties, linking momentum and wavelength.
The photon model therefore became the foundation for the broader framework of quantum mechanics, influencing Schrödinger’s wave equations and the probabilistic interpretation of matter.
Classical theory predicted that increasing light intensity should increase the energy of emitted electrons because wave energy was thought to be spread continuously.
However, experiments showed that intensity only affected the number of emitted electrons, not their maximum kinetic energy.
This inconsistency proved that energy transfer was not continuous but occurred in discrete quanta — each photon transferring its own energy to a single electron.
Practice Questions
Question 1 (2 marks)
State one piece of experimental evidence that supports the idea that light consists of photons, and explain how this evidence demonstrates the particulate nature of electromagnetic radiation.
Mark scheme:
• Identification of relevant experiment (1 mark) — e.g. photoelectric effect, blackbody radiation, or Compton scattering.
• Explanation of how the experiment shows photons have discrete energy (1 mark) — for example: electrons are emitted only when the incident frequency exceeds a threshold, showing that energy is transferred in quantised packets rather than continuously.
Question 2 (5 marks)
Describe how the understanding of electromagnetic radiation developed from the classical wave model to the photon model. In your answer, refer to experimental evidence and explain how this led to the acceptance of quantum ideas.
Mark scheme:
• Recognition that classical wave theory could not explain blackbody radiation or the photoelectric effect (1 mark).
• Description of Planck’s proposal that energy is quantised and emitted in discrete packets, E = hf (1 mark).
• Description of Einstein’s extension to the photoelectric effect — that light is made up of photons each with energy hf (1 mark).
• Reference to key experimental evidence confirming the photon model, e.g. Millikan’s verification or Compton scattering showing photon momentum (1 mark).
• Clear statement of how these experiments led to the acceptance of the photon model and the development of quantum physics (1 mark).

