OCR Specification focus:
‘Demonstrate photoelectric emission; evidence for the particulate nature of electromagnetic radiation.’
The photoelectric effect reveals how light interacts with matter, showing that electromagnetic radiation behaves as discrete energy packets called photons, not just continuous waves. This experiment fundamentally supports the quantum model of light.
The Photoelectric Effect: Core Observation
The photoelectric effect occurs when light of sufficient frequency shines onto a metal surface, causing electrons to be emitted. These emitted electrons are known as photoelectrons. The phenomenon provides direct evidence for the particulate nature of electromagnetic radiation, challenging classical wave theory predictions.
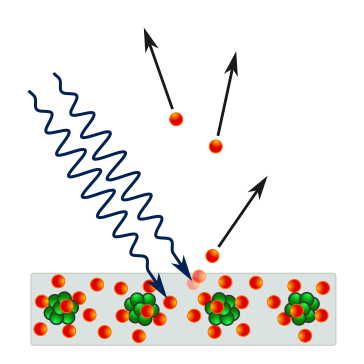
Photon–electron interaction at a surface: a high-frequency photon transfers energy to a surface electron, which escapes the material. The diagram emphasises the one-to-one interaction central to observing photoelectric emission. Source.
The experiment is typically demonstrated using an evacuated photoelectric cell consisting of:
A metal cathode exposed to incident light.
An anode connected in an electrical circuit to measure photocurrent.
A controllable potential difference (V) to investigate electron behaviour.
When light of frequency above a threshold value strikes the cathode, electrons are released and detected as a current. If the frequency is too low, no electrons are emitted, regardless of light intensity.
Classical Wave Theory Expectations
Before quantum physics, scientists believed light behaved purely as a wave. According to classical theory:
Increasing light intensity (brightness) should increase the energy transferred to electrons.
There should be a time delay before emission at low intensities, as electrons would need time to accumulate energy.
Emission should occur at any frequency, provided the intensity is sufficiently high.
However, experiments contradicted these expectations, revealing that light behaves differently at the atomic scale.
Experimental Observations
The photoelectric effect shows several key features inconsistent with wave theory:
1. Existence of a Threshold Frequency
No electrons are emitted unless the light frequency exceeds a specific threshold frequency (f₀), which depends on the metal’s work function. Below this frequency, even extremely intense light fails to produce emission.
2. Instantaneous Emission
Electrons are emitted immediately once the surface is illuminated with light above the threshold frequency, regardless of light intensity. There is no measurable delay, indicating that energy transfer occurs in discrete quanta, not gradually.
3. Dependence on Frequency, Not Intensity
The maximum kinetic energy of emitted photoelectrons depends only on the frequency of incident light, not its intensity.
Increasing intensity increases the number of electrons emitted per second, but not their individual energies.
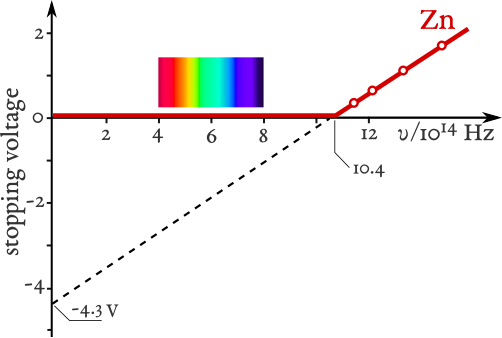
Graph of stopping potential versus light frequency for zinc. The line intercepts the frequency axis at the threshold frequency, where emission begins, and its slope reflects Planck’s constant (via Einstein’s photoelectric relation). This directly visualises frequency-dependent energy with no intensity dependence. Source.
These results could not be explained by wave theory but are perfectly consistent with the photon model of light.
Photon Model of Light
Albert Einstein proposed that electromagnetic radiation consists of discrete packets of energy called photons. Each photon carries a quantised amount of energy determined by its frequency.
EQUATION
—-----------------------------------------------------------------
Photon Energy (E) = hf
E = Energy of a single photon (joules, J)
h = Planck’s constant (6.63 × 10⁻³⁴ J·s)
f = Frequency of the electromagnetic radiation (hertz, Hz)
—-----------------------------------------------------------------
Each photon interacts with a single electron on the metal surface in a one-to-one interaction. If the photon’s energy exceeds the work function (the minimum energy needed to release an electron), emission occurs. Excess energy becomes the kinetic energy of the photoelectron.
Apparatus and Experimental Setup
A simple setup to observe photoelectric emission includes:
A vacuum photocell with a metal cathode and an anode.
A variable voltage supply connected across the electrodes.
A light source of adjustable frequency (often monochromatic).
An ammeter to detect current due to emitted photoelectrons.
A voltmeter to measure potential difference.
Procedure
Illuminate the metal cathode with light of a known frequency.
Gradually vary the applied voltage between anode and cathode
Measure the photocurrent as light frequency and intensity are changed.
Determine the threshold frequency where photoemission just ceases.
Observations
Emission occurs only for light frequencies above the threshold.
Increasing light intensity above this threshold increases current.
The stopping potential (voltage that just prevents photoelectrons from reaching the anode) remains constant for a given frequency but changes with frequency.
Evidence for the Particulate Nature of Light
The instantaneous emission and frequency dependence of photoelectrons strongly support the photon model. Each photon delivers energy hf to one electron. Below the threshold frequency, photons do not have sufficient energy to overcome the work function (Φ), regardless of intensity.
Work Function (Φ): The minimum energy required to release an electron from the surface of a material.
Since energy transfer happens in discrete quanta, not as a continuous wave, the photoelectric effect confirms that light exhibits particle-like behaviour. This discovery marked a significant step in the development of quantum physics.
Key Experimental Evidence Summarised
The main experimental findings that demonstrate photoelectric emission and support the photon model are:
No emission below a threshold frequency, proving light energy is quantised.
Instantaneous emission, showing energy transfer is direct and immediate.
Kinetic energy depends on frequency, not intensity, consistent with photon energy hf.
Emission rate depends on intensity, since more photons striking the surface release more electrons per second.
Practical Metals and Thresholds
Different metals have different threshold frequencies, corresponding to their work functions:
Sodium: threshold in the ultraviolet range.
Zinc: requires even higher frequencies.
Cesium and potassium: emit electrons under visible light, making them ideal for photoelectric cells.
These variations illustrate that the binding energy of electrons differs between materials, influencing the ease of photoemission.
Importance in Quantum Physics
Observing the photoelectric effect was pivotal in establishing the quantum theory of light. It provided experimental confirmation that electromagnetic radiation exhibits wave–particle duality, behaving as both a wave and a stream of particles depending on context. The success of Einstein’s photon explanation earned him the 1921 Nobel Prize in Physics and laid the foundation for modern quantum mechanics.
FAQ
Only electrons near the surface can escape because they experience less attraction from surrounding atoms.
Photons are absorbed within a very shallow region—typically a few nanometres deep—so the energy is transferred mainly to surface electrons.
Electrons deeper inside the metal lose energy through collisions before reaching the surface and therefore cannot overcome the work function, preventing their emission.
If a photon’s energy is less than the work function of the metal, it cannot eject an electron.
Possible outcomes include:
The photon being reflected or transmitted through the surface without interaction.
The photon’s energy being absorbed and converted into thermal energy, slightly heating the metal.
In either case, no photoelectrons are emitted because the photon–electron interaction is insufficient to overcome the binding energy.
Once the threshold frequency is exceeded, each photon can eject one electron.
Increasing intensity means more photons strike the surface per second.
Each photon can release one electron, so a higher photon rate produces a greater emission rate.
Below the threshold frequency, even if intensity is raised, photons still lack sufficient energy (hf < Φ), so no photoelectrons are emitted, and the current remains zero.
A clean, oxide-free surface is essential. Oxidation or contamination increases the effective work function, requiring higher photon energy for emission.
Tarnished or dirty surfaces can delay or prevent emission even when using normally adequate frequencies.
In precise experiments, metals like cesium or potassium are kept in vacuum conditions to prevent contamination.
Surface preparation directly affects the accuracy of determining the threshold frequency.
In principle, yes—provided the radiation’s frequency is high enough that the photon energy exceeds the metal’s work function.
For many metals, this lies in the ultraviolet or visible range.
For very large work functions, X-rays or even gamma rays might be needed.
Low-frequency radiation such as infrared or microwaves typically cannot cause emission because their photons have too little energy per quantum.
Practice Questions
Question 1 (2 marks)
Describe what is meant by the photoelectric effect and state one piece of evidence it provides for the particulate nature of electromagnetic radiation.
Mark scheme:
1 mark for stating that the photoelectric effect is the emission of electrons from a metal surface when light of sufficient frequency is incident on it.
1 mark for identifying evidence for the particle nature of light, e.g. emission only occurs above a threshold frequency or instantaneous emission even at low intensity.
Question 2 (5 marks)
A student investigates the photoelectric effect using light of different frequencies on a clean metal surface.
(a) Describe how the student could use a photocell and an adjustable potential difference to demonstrate the existence of a threshold frequency. (3 marks)
(b) Explain why increasing the intensity of light below the threshold frequency does not result in photoelectron emission. (2 marks)
Mark scheme:
(a)
1 mark: Mentions setting up a photocell with a metal cathode and anode connected through a variable potential difference and ammeter.
1 mark: Describes illuminating the cathode with light of increasing frequency while measuring the photocurrent or observing when current first appears.
1 mark: Identifies that no electrons are emitted below a certain frequency, showing a threshold frequency exists for emission.
(b)
1 mark: Explains that light intensity affects the number of photons, not their individual energies.
1 mark: States that if the photon energy (hf) is less than the work function, no electrons are emitted regardless of intensity.

