AP Syllabus focus:
‘Explain the mechanism of dehydration synthesis, including removal of hydrogen and hydroxyl groups, release of water, and covalent bonding of monomers into polymers.’
Dehydration synthesis is the core chemical strategy cells use to build large biological molecules from small subunits. Understanding the stepwise mechanism clarifies how polymers form, how water is produced, and why enzymes are essential.
Key Terms and What is Being Built
Dehydration synthesis (condensation reaction): A reaction that links two molecules by forming a covalent bond while removing an H from one and an OH from the other, producing water.
Dehydration synthesis is typically used to assemble repeating subunits into larger structures through repeated covalent-bond formation.
Polymerization: The process of forming a polymer by covalently joining many monomers in a chain or network through repeating reaction steps.
In biology, polymerization commonly builds long, information-rich or function-rich macromolecules by adding monomers in a controlled order.
Step-by-Step Mechanism of Dehydration Synthesis
1) Monomers are Positioned and Oriented
For dehydration synthesis to occur, two monomers must be brought close enough that specific functional groups can react.
Reactive sites are typically functional groups (for example, hydroxyl, carboxyl, amine, phosphate).
Cells rely on enzymes to hold substrates in the correct orientation, increasing reaction rate and reducing incorrect linkages.
2) Removal of Hydrogen and Hydroxyl Groups
The defining chemical event is removal of the components of water from the two reacting monomers.
One monomer contributes a hydrogen (H).
The other monomer contributes a hydroxyl group (OH).
These combine to form a molecule of water (H₂O) as a product.
This is why the process is called dehydration: water is formed by “dehydrating” the reacting pair.
3) Covalent Bond Formation Links Monomers into a Growing Polymer
As H and OH are removed, electrons are rearranged and a new covalent bond forms between the monomers, producing a larger molecule.
The newly formed covalent bond becomes part of the polymer backbone or linkage region.
Repeating the same mechanism over and over extends the polymer, one linkage at a time.
Different monomer chemistries yield different covalent linkages, but the mechanistic theme is consistent: remove H and OH → release water → form covalent bond.
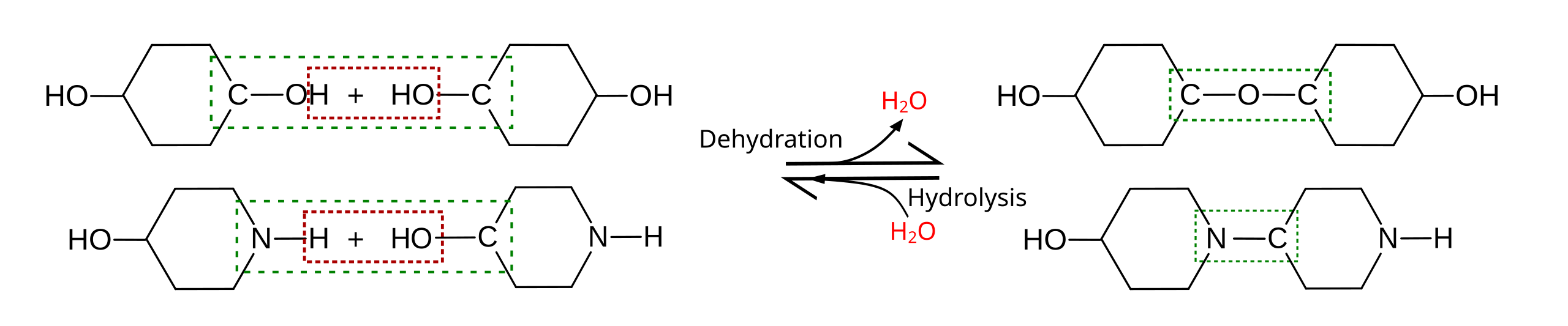
This diagram summarizes dehydration (condensation) versus hydrolysis as opposite reactions. It explicitly shows how an -H and an -OH are removed to form H₂O while a new covalent linkage forms between the two subunits (and how adding water reverses the bond). Use it as a visual checklist for the AP mechanism: hydrogen removal, hydroxyl removal, water release, and covalent bonding. Source
Examples of linkage types produced by dehydration synthesis include peptide, glycosidic, phosphodiester, and ester bonds.
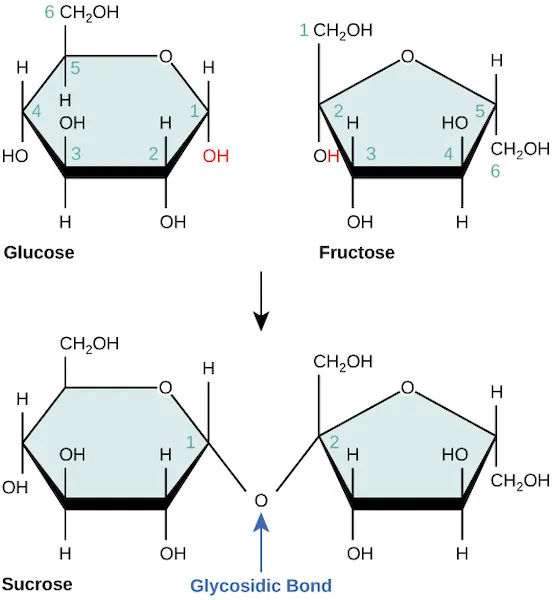
This textbook figure shows dehydration synthesis forming a glycosidic bond during sucrose formation. The hydroxyl group from one monosaccharide and a hydrogen from the other combine to produce water, while the remaining atoms form the covalent linkage between the rings. It’s a specific example of how the same dehydration mechanism builds carbohydrate polymers from monomers. Source
How Polymerization Proceeds in Cells
Repetition, Directionality, and Control
Polymer growth is not random in living systems; it is regulated so that specific sequences or architectures form.
Polymerization often occurs as a repeated cycle: bind monomer(s) → catalyse dehydration → release product → repeat.
Control mechanisms ensure the correct monomer is added and that growth occurs at the correct reactive end or site (depending on the polymer system).
Enzymes Make Dehydration Synthesis Biologically Feasible
Although dehydration synthesis can occur in nonliving chemistry, biological polymerization depends on catalysis.
Enzymes lower activation energy, align substrates, and stabilise transition states.
Enzymes help prevent side reactions (for example, incorrect bonding between the wrong groups).
Many polymer-forming enzymes couple bond formation to other favourable processes (often involving energy-carrying molecules) to drive polymer growth efficiently.
Water Production and Reversibility Considerations
Because water is a product, dehydration synthesis is sensitive to cellular conditions.
The immediate release (or removal) of products, including water and the growing polymer, can help the reaction proceed forward.
In principle, the reverse reaction is possible, so cells maintain conditions and enzyme control that favour polymer formation when building macromolecules.
The mechanism required for AP Biology can be tracked directly in every cycle: hydrogen removal, hydroxyl removal, water release, and covalent bonding that converts monomers into polymers.
FAQ
In cells it is often multi-step at the enzyme active site, with transient enzyme–substrate interactions that effectively create short-lived intermediates before the final covalent bond forms.
They enforce specificity by binding substrates in fixed orientations and by shaping the active site to favour one reaction pathway over competing side reactions.
Cells frequently couple bond formation to energetically favourable reactions (for example, breaking high-energy bonds) to make polymer growth proceed reliably under cellular conditions.
Typically it does not accumulate at the reaction site because diffusion and cellular water balance disperse it; local product removal can help favour continued bond formation.
Yes. Branching occurs when monomers have multiple reactive groups; branching patterns are controlled by enzymes that select which specific functional group participates in bond formation.
Practice Questions
Describe what is removed from each of two monomers during dehydration synthesis and name the molecule released. (2 marks)
Hydrogen (H) is removed from one monomer (1)
Hydroxyl group (OH) is removed from the other, forming/releasing water (H2O) (1)
Explain how dehydration synthesis produces a polymer from monomers. In your answer, include the roles of H and OH removal, water release, and covalent bond formation. (5 marks)
Monomers are joined together to build a larger molecule/polymer (1)
An H is removed from one monomer (1)
An OH is removed from the other monomer (1)
H and OH combine to form/release water (1)
A covalent bond forms between the monomers, and repetition of this step extends the polymer (1)

