AP Syllabus focus:
‘Describe how dehydration synthesis and hydrolysis are complementary chemical reactions that build and break down biological macromolecules.’
Biological macromolecules are assembled and disassembled through predictable reactions involving water. Understanding how cells build polymers from monomers and then break them apart is essential for explaining digestion, biosynthesis, and energy flow.
Core Idea: Polymers are Dynamic
Cells constantly balance building and breaking large molecules to meet changing needs (growth, storage, repair, and energy release). Two reactions form a complementary pair:
Dehydration synthesis (condensation) builds polymers by forming covalent bonds and producing water.
Hydrolysis breaks polymers by using water to cleave covalent bonds.
Key terms
Polymer: A large molecule made of many repeating monomer subunits covalently bonded together.
This concept applies broadly to biological macromolecules; the key AP focus here is the reaction logic of adding or removing water to shift between monomers and polymers.
Dehydration Synthesis (Condensation): Building Polymers
Dehydration synthesis links monomers together by forming a new covalent bond while removing the components of a water molecule.
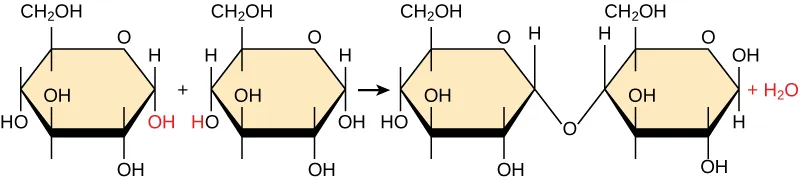
Two glucose monomers undergo a condensation (dehydration synthesis) reaction to form the disaccharide maltose. The diagram highlights that an -H from one monomer and an -OH from the other combine to produce , while the remaining atoms become linked by a new covalent bond. Source
What Happens Chemically
A bond forms between two monomers.
An -H is removed from one monomer and an -OH is removed from the other.
These removed groups combine to form H₂O, which is released as a product.
The monomers become covalently linked, extending a polymer.
Why Cells Use It
It enables the orderly construction of large biological molecules from smaller building blocks.
The covalent bonds created store chemical energy and structural information that can be accessed later.
Because building polymers is not automatically favourable in water-rich environments, cells typically rely on enzymes to speed the reaction and control when and where it occurs.
What to Remember for AP Biology
“Dehydration” refers to the loss of water from the reacting monomers (water is produced, not consumed).
Each time a new linkage forms, one water molecule is produced (conceptually, one per bond formed).
Hydrolysis: Breaking Polymers
Hydrolysis reverses dehydration synthesis by breaking a covalent bond in a polymer through the addition of water.
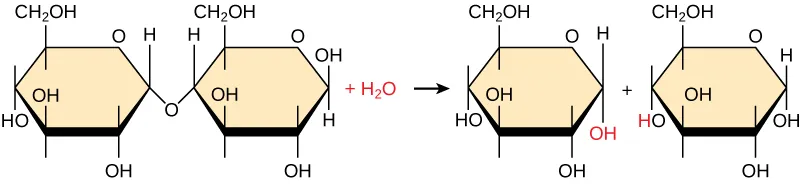
Maltose is broken into two glucose monomers by hydrolysis, with water acting as a reactant rather than a product. The figure emphasizes that splits into -H and -OH, which attach to the exposed ends after the covalent bond is cleaved. Source
What Happens Chemically
A water molecule is split into -H and -OH.
These groups attach to the exposed ends created when a bond breaks.
The polymer becomes shorter, ultimately yielding smaller molecules or individual monomers.
Why Cells Use It
It allows organisms to digest large molecules into absorbable subunits.
It supports cellular recycling: damaged or unneeded polymers can be dismantled so monomers can be reused.
It can rapidly mobilise stored chemical energy and materials when demand increases.
What to Remember for AP Biology
Hydrolysis requires water as a reactant.
It is the key reaction type for breaking down biological macromolecules during processes like digestion and intracellular turnover.
Complementary Relationship: a Reversible Logic
Dehydration synthesis and hydrolysis are described as complementary because they are chemically opposite in how they handle water and covalent bonds.
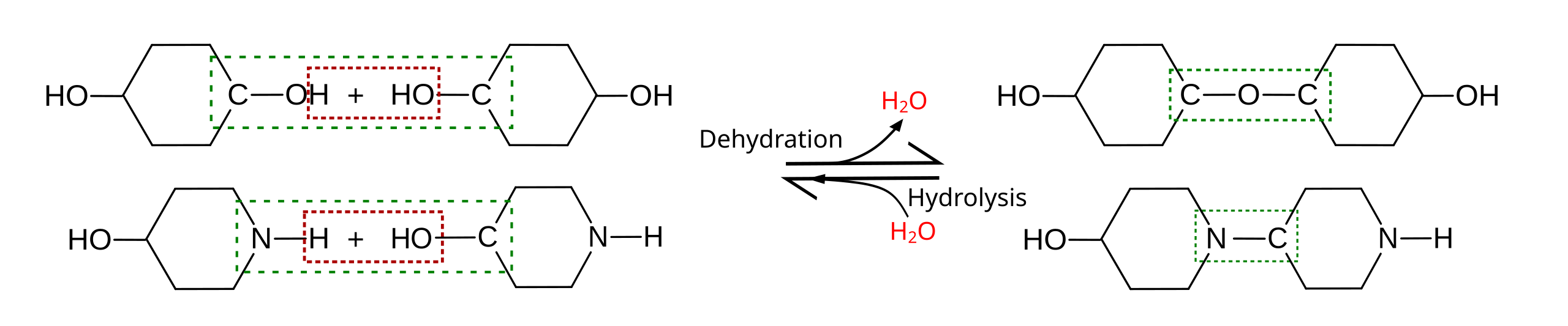
A generalized schematic comparing dehydration reactions (bond formation with water produced) and hydrolysis reactions (bond cleavage with water consumed). By showing where -H and -OH move in each direction, the diagram makes the “reversible logic” between monomers and polymers explicit. Source
How They Complement Each Other
Bond status
Dehydration synthesis: forms covalent bonds between subunits.
Hydrolysis: breaks covalent bonds between subunits.
Role of water
Dehydration synthesis: water is produced.
Hydrolysis: water is consumed.
Biological outcome
Dehydration synthesis: converts monomers → polymers (building).
Hydrolysis: converts polymers → monomers (breaking).
Direction Depends on Cellular Needs
Cells regulate these opposing processes so that polymer formation does not occur indiscriminately and polymer breakdown does not destroy needed structures. Enzymes provide specificity, ensuring the correct bonds are formed or cleaved in the correct context.
FAQ
Not usually at a useful rate. Cells rely on specific enzymes to lower activation energy and to ensure the correct monomers are joined in the correct order and location.
Water provides a simple chemical “switch”:
removing H and OH helps create a new linkage
adding H and OH helps restore separated subunits
This makes the two reactions naturally reversible in logic.
Conceptually, yes per bond formed or broken. In long polymers, many bonds are involved, so the total water molecules produced/consumed scales with the number of linkages made/cleaved.
They provide specificity by binding particular substrates and positioning bonds for cleavage. Without the correct enzyme, many biological covalent bonds are stable enough to persist in watery environments.
Yes. Different pathways and compartments can build some polymers while others are being broken down, allowing tight control of resources and rapid responses to changing cellular demands.
Practice Questions
State how dehydration synthesis and hydrolysis differ in their use of water and their effect on covalent bonds. (2 marks)
Dehydration synthesis produces/removes water and forms a covalent bond (1)
Hydrolysis uses/adds water and breaks a covalent bond (1)
Explain why dehydration synthesis and hydrolysis are described as complementary reactions in biological systems, and relate this to polymer formation and breakdown. (5 marks)
Dehydration synthesis links monomers to form polymers (1)
A covalent bond is formed during dehydration synthesis (1)
Water is produced during dehydration synthesis (1)
Hydrolysis breaks polymers into smaller molecules/monomers (1)
Water is used/consumed during hydrolysis to break the covalent bond (1)

