AP Syllabus focus:
‘Describe how hydrolysis uses water to cleave covalent bonds in polymers, splitting them into smaller molecules or individual monomers.’
Hydrolysis is the central chemical strategy cells use to digest food, recycle biomolecules, and remodel cellular structures. Understanding how water participates in bond cleavage clarifies how large macromolecules are converted into usable, smaller building blocks.
Core Idea: Using Water to Break Polymers
Macromolecules such as polysaccharides and proteins are often polymers built from repeating monomers linked by covalent bonds. Hydrolysis is the reaction that reverses polymer formation by using water to cleave covalent bonds, producing smaller molecules or individual monomers.
Hydrolysis: a chemical reaction in which water is used to break a covalent bond, typically splitting a polymer into shorter units by adding H and OH across the bond.
The key chemical logic is that the water molecule is consumed: its atoms become part of the products, rather than simply surrounding the reaction.
What Happens to the Water Molecule During Hydrolysis?
During hydrolysis, the bond between two subunits is broken and the components of water are added to the exposed ends:
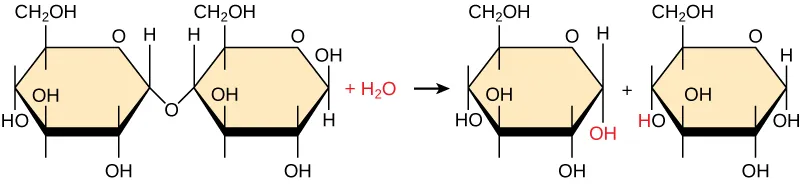
Hydrolysis of the disaccharide maltose into two glucose monomers using water. The diagram emphasizes that is split so that one product gains H and the other gains OH, illustrating how water is consumed and becomes incorporated into the products. Source
The water molecule is split into H and OH
H attaches to one fragment
OH attaches to the other fragment
The result is two smaller molecules that are chemically “capped” and more stable in solution
This is why hydrolysis is well-suited to turning a large, connected polymer into many soluble, transportable subunits.
= number of covalent bonds cleaved (dimensionless)
= water molecules consumed to cleave those bonds (molecules)
In biological systems, the “smaller units” commonly become the monomers that can be reused for synthesis or catabolised for energy.
Enzymes and Specificity in Biological Hydrolysis
Although hydrolysis is chemically straightforward, cells rely on enzymes to make it fast and specific under mild conditions (physiological temperature and pH).
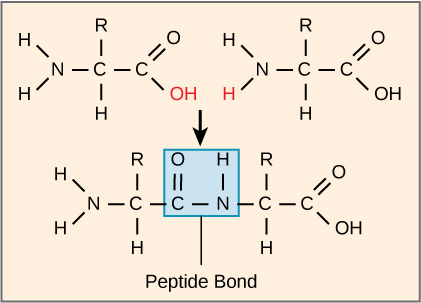
Formation of a peptide bond between two amino acids shown as a dehydration (condensation) reaction, with water produced from an –OH and an –H. Because hydrolysis is the reverse reaction, this diagram helps you localize the covalent bond that hydrolases ultimately cleave and where H/OH end up when water is added back across that bond. Source
Hydrolase: an enzyme that catalyses bond cleavage by adding water, lowering activation energy and increasing reaction rate.
Enzyme-controlled hydrolysis matters because cells must:
Target the correct bond (high substrate specificity)
Control timing and location (e.g., digestion vs intracellular recycling)
Prevent uncontrolled breakdown of essential cellular polymers
Hydrolysis as “Breakdown” of Macromolecules in Organisms
Hydrolysis supports biological breakdown in two major contexts:
Digestive Breakdown (Extracellular or Lumen-Based)
Many organisms hydrolyse dietary polymers into absorbable subunits:
Large macromolecules are too big to cross membranes efficiently
Hydrolysis generates smaller molecules that can be transported into cells
Products become substrates for metabolism or rebuilding body polymers
Cellular Recycling (Intracellular)
Cells also hydrolyse their own macromolecules to maintain homeostasis:
Damaged or misfolded polymers can be dismantled into reusable components
Controlled hydrolysis helps regulate the size and abundance of biomolecules
Monomers can be redirected into new synthesis pathways when resources are limited
Outcomes of Hydrolysis: Smaller Molecules or Individual Monomers
Hydrolysis does not always reduce a polymer all the way to monomers in one step. Depending on conditions and enzymes, products may include:
Oligomers (short chains)
Individual monomers
Mixed fragments of different lengths
Biologically, this flexibility allows staged processing: partial hydrolysis can prepare molecules for further breakdown, transport, or regulated remodelling.
Why “Cleaving Covalent Bonds” is a Big Deal
Because the links between monomers are covalent, they are relatively stable. Hydrolysis is therefore a key gateway reaction that:
Converts stable, information- or structure-rich polymers into mobile units
Changes physical properties (often increasing solubility and decreasing size)
Enables the flow of matter through biological systems by making subunits available for uptake and reuse
FAQ
Hydrolysis can be energetically favourable or unfavourable depending on the specific bond and context. Cells often couple reactions or use enzymes to control rate rather than “force” spontaneity.
pH can change enzyme shape and active-site charge, altering catalysis. Extreme pH can also promote non-enzymatic hydrolysis, but this is usually too slow or damaging for cells.
Covalent bonds have activation energy barriers. Water alone typically cannot break these bonds quickly at physiological conditions without catalysis.
Enzyme type, substrate accessibility, and reaction time matter. Some enzymes remove end subunits, while others cut internally, producing mixtures of fragment lengths.
Compartmentalisation, enzyme regulation (activation/inhibition), and selective targeting of substrates help restrict hydrolysis to appropriate molecules, times, and locations.
Practice Questions
Explain how hydrolysis breaks a polymer into smaller molecules. (2 marks)
States that water is used/consumed in the reaction (1)
States that a covalent bond between subunits is cleaved and H and OH are added to the products (1)
A student claims that “adding water to a polymer always makes it dissolve but does not change the polymer itself.” Assess this claim using principles of hydrolysis. (5 marks)
Identifies that hydrolysis is a chemical reaction that breaks covalent bonds, not just a physical mixing process (1)
Explains that water is split into H and OH which become part of the products (1)
States that the polymer is converted into smaller molecules/monomers (1)
Links smaller size/changed end groups to altered properties such as increased solubility and transportability (1)
Notes that enzymes (hydrolases) typically catalyse hydrolysis in biological systems to control the reaction (1)

