IB Syllabus focus:
‘Oceans dissolve CO₂ and also outgas it; fossil-fuel emissions outpace uptake. Increased dissolved CO₂ lowers pH, impairing calcium carbonate formation in corals and molluscs.’
The world’s oceans regulate climate by absorbing atmospheric carbon dioxide, but rising fossil-fuel emissions have overwhelmed this natural balance, driving ocean acidification and threatening marine ecosystems.
Oceans as Carbon Reservoirs
The oceans act as vast carbon sinks, dissolving significant amounts of atmospheric carbon dioxide (CO₂). This process moderates climate change by reducing greenhouse gas concentrations. However, the capacity of oceans to absorb CO₂ is limited, and they also naturally outgas CO₂ back to the atmosphere, maintaining a dynamic equilibrium.
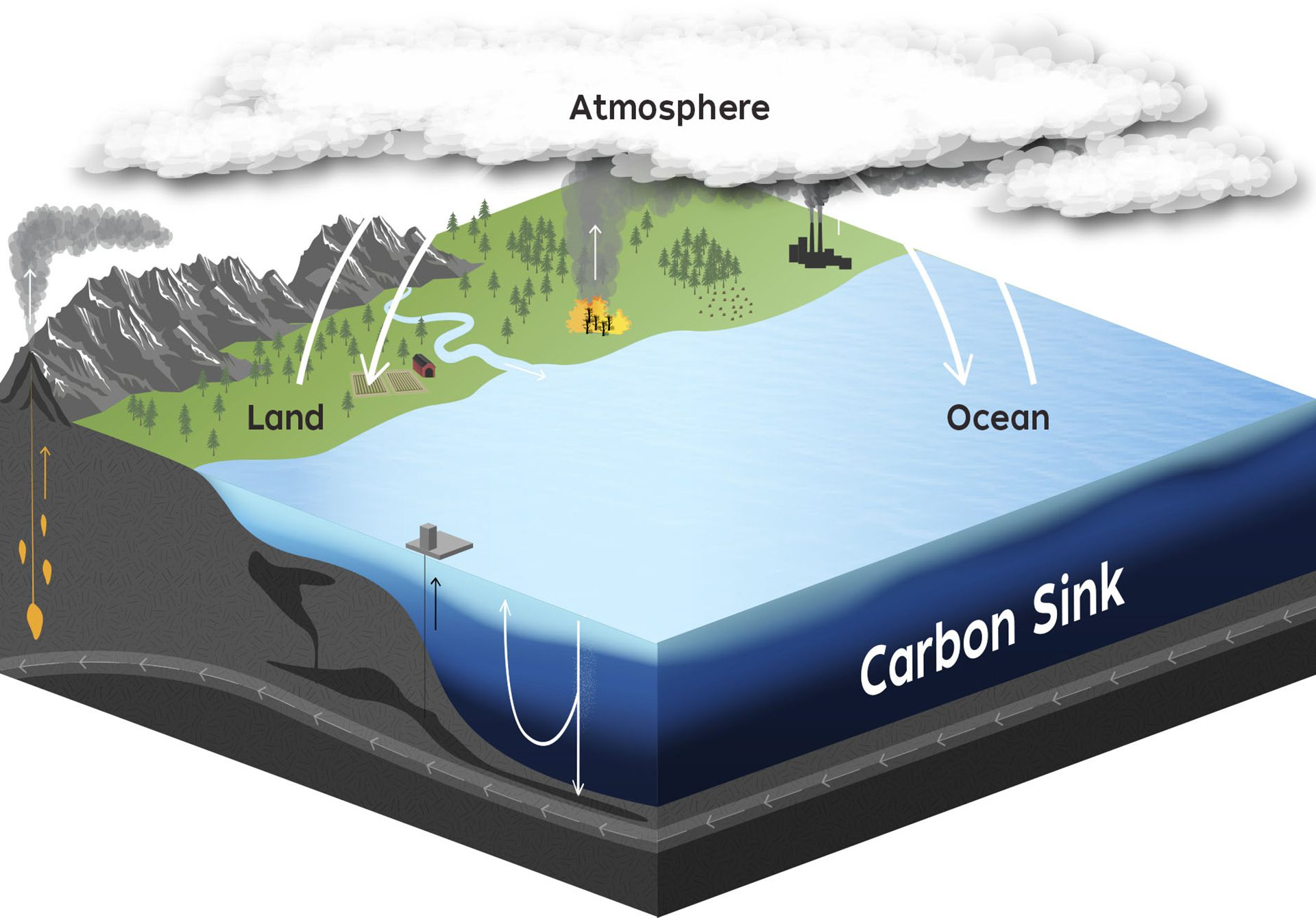
Illustration of carbon exchange among atmosphere, surface ocean (uptake and release), and the deeper ocean, highlighting the ocean’s role as a carbon sink. While the figure also depicts land fluxes, it is used here specifically to visualise dissolution and outgassing at the sea surface. Source.
Ocean Acidification
When CO₂ dissolves in seawater, it reacts with water to form carbonic acid (H₂CO₃).
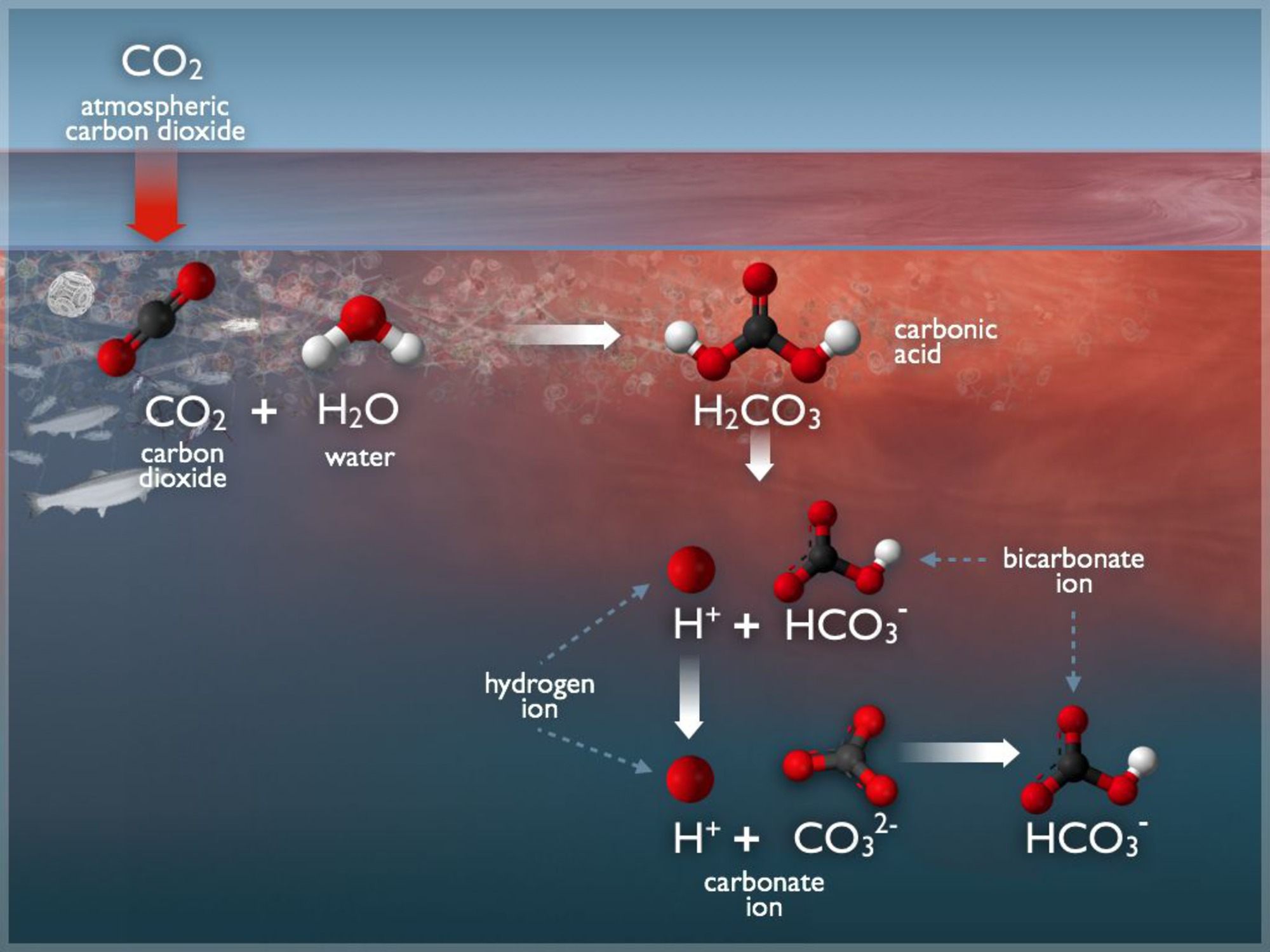
Diagram showing atmospheric CO₂ entering surface seawater and reacting to form carbonic acid (H₂CO₃), which dissociates to H⁺ and HCO₃⁻; additional H⁺ drives a shift that lowers CO₃²⁻ availability. Labels clearly identify each species and step. This directly illustrates the mechanism of ocean acidification described in the IB syllabus. Source.
This weak acid dissociates, releasing hydrogen ions (H⁺), which lower ocean pH.
Ocean Acidification: The ongoing decrease in ocean pH caused by the absorption of anthropogenic CO₂, leading to more acidic conditions in marine environments.
The pre-industrial ocean pH averaged about 8.2. Today, it has fallen to roughly 8.1, representing a 30% increase in acidity. This change, though seemingly small, significantly alters marine chemistry.
Chemical Reactions Involved
The absorption of CO₂ drives a series of transformations:
CO₂ + H₂O → H₂CO₃ (carbonic acid)
H₂CO₃ ⇌ H⁺ + HCO₃⁻ (bicarbonate ion)
HCO₃⁻ ⇌ H⁺ + CO₃²⁻ (carbonate ion)
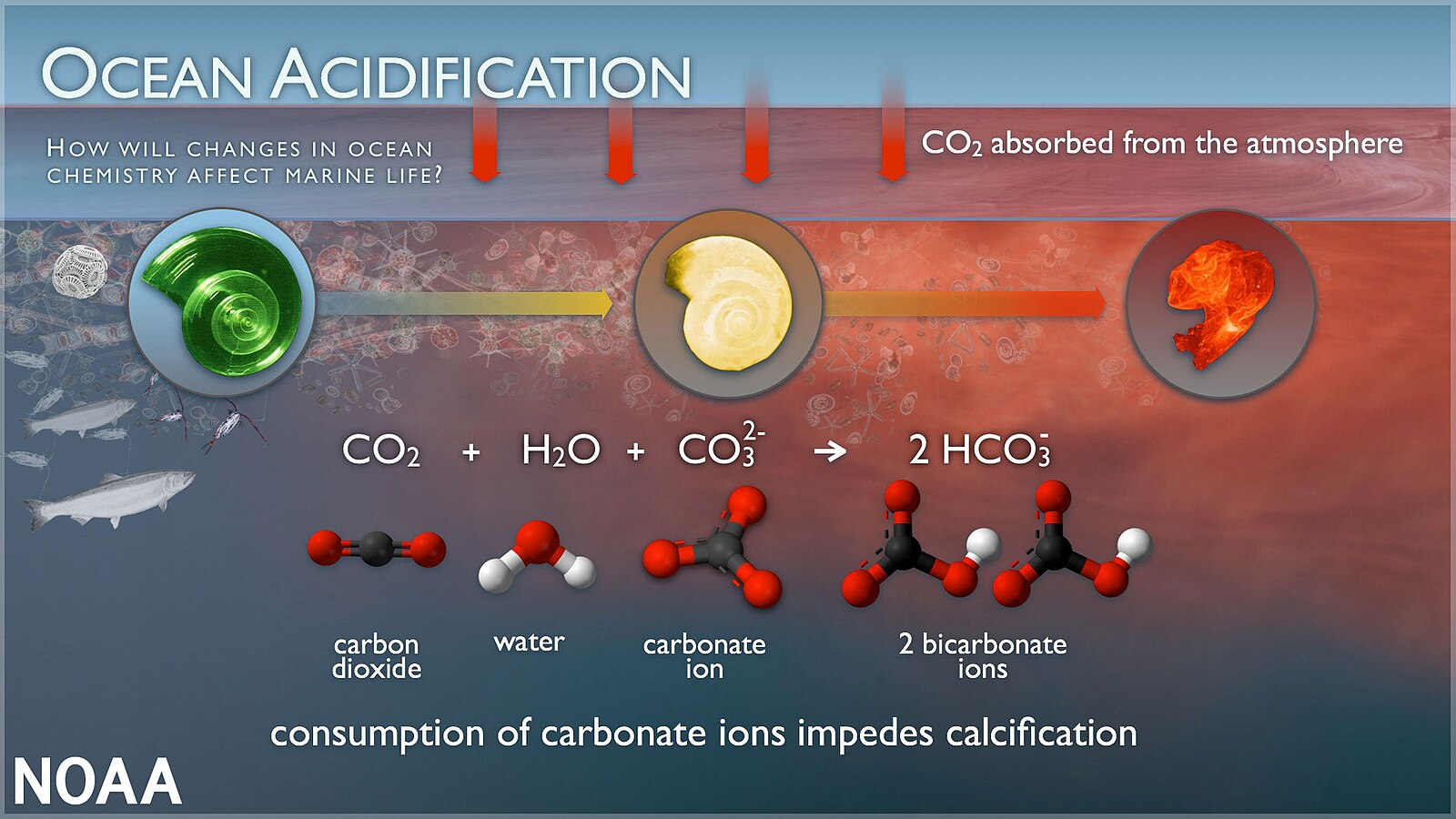
As hydrogen ions increase, carbonate ions (CO₃²⁻) become depleted, directly affecting organisms that rely on them.
Impacts on Marine Life
Calcium Carbonate Formation
Many marine organisms, including corals, molluscs, and some plankton, build shells or skeletons from calcium carbonate (CaCO₃). Reduced carbonate availability hinders this process.
Paired photographs of a pteropod shell show intact structure versus dissolution under lower-pH conditions, demonstrating reduced calcification and increased erosion. This is a clear, organism-level consequence of acidification that underpins reef and food-web vulnerabilities. Source.
Calcium Carbonate Saturation State: The measure of how easily marine organisms can form CaCO₃ structures; a decline signals greater difficulty in shell and skeleton building.
Key Consequences
Coral reefs: Slower growth rates, weakened structures, and greater vulnerability to erosion.
Shellfish: Thinner, more fragile shells in clams, oysters, and mussels.
Plankton: Reduced calcification in coccolithophores, weakening the base of marine food webs.
Ecosystem and Biodiversity Effects
Ocean acidification can trigger cascading impacts:
Coral reef decline reduces biodiversity, as reefs shelter about 25% of marine species.
Food chain disruptions affect fisheries, threatening global food security.
Declining populations of calcifying organisms may destabilise entire marine ecosystems.
Regional Variability
Acidification is not uniform across the globe.
Polar oceans: Naturally low carbonate concentrations make them especially vulnerable.
Upwelling regions: Coastal areas where deep, CO₂-rich waters rise to the surface experience intensified acidification.
Tropical reefs: Already stressed by warming and pollution, these ecosystems face compounded risks.
Link to Human Activities
Burning fossil fuels, deforestation, and industrial processes have sharply increased atmospheric CO₂, outpacing the ocean’s natural buffering capacity. Since the Industrial Revolution, oceans have absorbed about 30–40% of anthropogenic CO₂, shifting chemical balances faster than ecosystems can adapt.
Wider Implications for Biosphere Integrity
Ocean acidification is a threat to biosphere integrity, one of the identified planetary boundaries. As ecosystems degrade, the resilience of the biosphere declines, increasing the risk of crossing ecological tipping points. This endangers not just marine life but also human societies dependent on ocean resources.
Monitoring and Research Approaches
Measuring Ocean Acidification
pH sensors and probes track changes in real time.
Carbonate chemistry sampling monitors levels of CO₂, bicarbonate, and carbonate ions.
Long-term time-series studies help scientists assess trends and predict impacts.
Predictive Modelling
Models simulate future scenarios of CO₂ emissions and ocean chemistry. These projections indicate that without mitigation, ocean pH could decline by an additional 0.3–0.4 units by 2100.
Mitigation Strategies
While the IB syllabus highlights the problem rather than solutions in detail, it is important to note key approaches:
Reducing fossil-fuel emissions through renewable energy and efficiency.
Protecting and restoring marine ecosystems such as seagrass meadows and mangroves that absorb carbon.
Limiting other stressors, such as overfishing and pollution, to build ecosystem resilience.
FAQ
Ocean acidification progresses faster in regions where natural conditions already limit carbonate availability. Polar oceans are particularly sensitive because cold water holds more dissolved CO₂, and upwelling zones bring carbon-rich deep water to the surface. These processes create “hotspots” of acidification, where organisms face chemical stress sooner than in other parts of the world.
While acidification lowers carbonate availability, warmer waters compound stress by reducing oxygen levels and increasing coral bleaching risk. Together, these stressors weaken marine organisms’ ability to adapt. For example, corals under heat stress are less able to allocate energy to skeleton building, making them doubly vulnerable to reduced carbonate levels.
Plankton such as coccolithophores build microscopic calcium carbonate plates. If acidification limits their calcification, plankton populations decline or change composition. This directly impacts higher trophic levels like fish and whales, since plankton form the base of marine food webs and are critical to global carbon cycling.
Unlike atmospheric CO₂, which can stabilise relatively quickly, ocean chemistry responds more slowly. Some buffering occurs naturally through dissolution of minerals from seafloor sediments, but this process takes centuries to millennia. Therefore, while emission reductions can halt further acidification, reversing existing pH changes will require very long timescales.
Marine organisms use different crystalline forms of calcium carbonate—aragonite and calcite. Aragonite, used by corals and pteropods, is more soluble and dissolves more readily in acidic conditions than calcite, used by many other molluscs. As a result, species relying on aragonite are among the first to experience severe impacts.
Practice Questions
Question 1 (2 marks)
Explain why increasing atmospheric carbon dioxide leads to lower pH levels in the oceans.
Mark Scheme
1 mark: States that dissolved CO₂ forms carbonic acid in seawater.
1 mark: Explains that carbonic acid dissociates to release hydrogen ions, which lower pH.
Question 2 (5 marks)
Discuss the impacts of ocean acidification on marine organisms that depend on calcium carbonate, and outline how this may affect marine ecosystems.
Mark Scheme
1 mark: Identifies that reduced carbonate ion availability makes it harder for organisms to form calcium carbonate shells/skeletons.
1 mark: Names at least one affected group (e.g., corals, molluscs, plankton).
1 mark: Explains an organism-level consequence (e.g., weaker shells, slower reef growth).
1 mark: Describes a broader ecological consequence such as loss of biodiversity or disruption of food chains.
1 mark: Links these changes to ecosystem-level impacts, such as reduced resilience or threats to fisheries.

