IB Syllabus focus:
‘Organic stores in biomass and detritus; inorganic in atmosphere, soils, waters. Microbes drive fixation (N₂→NH₃), nitrification (NH₃/NO₂⁻→NO₃⁻), denitrification (NO₃⁻→N₂), and decomposition (organic N→NH₄⁺).’
The nitrogen cycle is a fundamental ecological process, maintaining life by circulating nitrogen between organic and inorganic forms. Microbes play critical roles, ensuring ecosystem productivity and stability.
Nitrogen Stores
Nitrogen is an essential macronutrient required for the synthesis of proteins, nucleic acids, and chlorophyll. It is stored in both organic and inorganic forms.
Organic Nitrogen Stores
Organic nitrogen is stored in:
Biomass: All living organisms contain nitrogen within proteins and nucleic acids.
Detritus: Dead organic matter, such as fallen leaves, animal remains, and faeces, which later undergo decomposition.
Inorganic Nitrogen Stores
Inorganic nitrogen exists in several environmental compartments:
Atmosphere: Nitrogen gas (N₂) makes up about 78% of the atmosphere but is unavailable directly to most organisms.
Soils: Contain compounds like ammonium (NH₄⁺), nitrate (NO₃⁻), and nitrite (NO₂⁻).
Water bodies: Aquatic systems hold dissolved inorganic nitrogen compounds, influencing productivity.
Nitrogen Store: A reservoir where nitrogen is held in either organic or inorganic forms within ecosystems.
Microbial Processes in the Nitrogen Cycle
Microbes mediate the transformation of nitrogen between different chemical forms, ensuring availability to organisms and regulating ecosystem balance.
Nitrogen Fixation
This process converts inert atmospheric nitrogen (N₂) into biologically usable forms such as ammonia (NH₃).
Key microbes:
Nitrogen-fixing bacteria (e.g., Rhizobium) in symbiotic relationships with legumes.
Free-living soil bacteria (e.g., Azotobacter).
Nitrogen Fixation: The process by which atmospheric nitrogen gas (N₂) is converted into ammonia (NH₃) by specialised microorganisms.
Nitrification
This is the two-step oxidation of ammonium (NH₄⁺) into nitrate (NO₃⁻), an important form assimilated by plants.
Steps:
Ammonium (NH₄⁺) → Nitrite (NO₂⁻) by Nitrosomonas bacteria.
Nitrite (NO₂⁻) → Nitrate (NO₃⁻) by Nitrobacter bacteria.
Nitrification: A microbial process where ammonium is sequentially oxidised into nitrite and then nitrate.
Denitrification
This reduces nitrates back to atmospheric nitrogen (N₂), closing the cycle. It usually occurs in anaerobic conditions such as waterlogged soils.
Key microbes:
Pseudomonas denitrificans
Paracoccus denitrificans
Denitrification: The microbial reduction of nitrates (NO₃⁻) into gaseous nitrogen (N₂), returning it to the atmosphere.
Decomposition and Ammonification
When plants and animals die, decomposers such as fungi and bacteria break down organic nitrogen into simpler compounds.
Proteins and nucleic acids in detritus are converted into ammonium (NH₄⁺).
This ammonium may then re-enter the nitrification process.
Ammonification: The conversion of organic nitrogen compounds into ammonium (NH₄⁺) by decomposer organisms.
Microbes drive fixation (N₂→NH₃), nitrification (NH₃/NO₂⁻→NO₃⁻), denitrification (NO₃⁻→N₂), and decomposition (organic N→NH₄⁺).
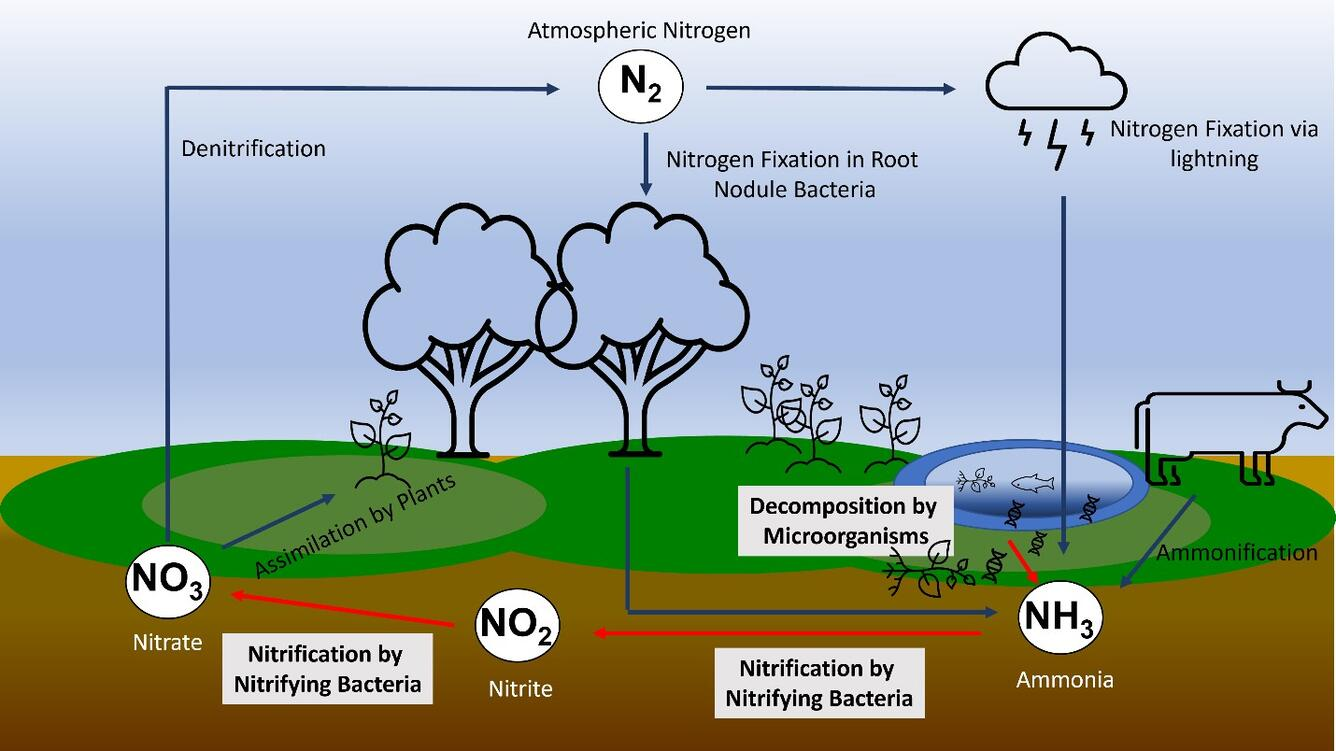
Schematic overview of the nitrogen cycle with microbially mediated steps clearly indicated, reinforcing how fixation, ammonification, nitrification, and denitrification transfer nitrogen among stores. Labels are concise and legible for rapid revision. Source.

Root nodules on white clover showing the pink hue of leghaemoglobin in active, oxygen-buffered nodules that host Rhizobium. This links microbial fixation directly to plant biomass as an organic nitrogen store. Source.
Importance of Microbes in Nitrogen Cycling
Microbes maintain the nitrogen balance, preventing both shortages and toxic accumulations. Their activities are vital for:
Supporting plant growth by providing nitrate and ammonium.
Sustaining productivity in aquatic and terrestrial ecosystems.
Balancing global nitrogen levels, linking biosphere and atmosphere.
Human Impact on Microbial Nitrogen Cycling
Human activities alter microbial nitrogen processes:
Fertiliser use introduces large amounts of fixed nitrogen, bypassing natural fixation.
Water pollution arises from nitrate leaching, promoting eutrophication.
Soil disturbance can reduce microbial diversity and disrupt balance between nitrification and denitrification.
Summary of Key Microbial Roles
Nitrogen-fixing bacteria: Convert N₂ into NH₃.
Nitrifying bacteria: Convert NH₄⁺ → NO₂⁻ → NO₃⁻.
Denitrifying bacteria: Convert NO₃⁻ back to N₂.
Decomposers: Break down organic matter into NH₄⁺.
Together, these processes ensure nitrogen is continuously cycled between the atmosphere, soils, waters, and living organisms, making it accessible for ecosystems to thrive.
FAQ
Nitrogen-fixing bacteria thrive in soils with moderate moisture and good aeration. Oxygen levels must be regulated, as too much oxygen inhibits the activity of nitrogenase, the key enzyme in fixation.
Legume-associated bacteria are particularly effective in neutral pH soils with sufficient phosphorus and iron, which support both plant growth and microbial metabolism.
Nitrifying bacteria are obligate aerobes, requiring oxygen to oxidise ammonium into nitrite and nitrate. When soils are compacted or waterlogged, oxygen levels fall, limiting their activity.
These bacteria also grow slowly compared to other microbes, meaning physical disturbance or chemical changes (such as pesticide use) can suppress their populations for extended periods.
While denitrification usually produces harmless nitrogen gas (N₂), it can also release nitrous oxide (N₂O), a potent greenhouse gas.
This often occurs when soils are waterlogged and oxygen is scarce. Incomplete denitrification under these conditions promotes N₂O release instead of complete reduction to N₂.
Ammonium (NH₄⁺) is a key intermediate between organic matter and plant-available nitrogen.
It is directly taken up by some plants, particularly in acidic soils.
It serves as the starting point for nitrification, which converts ammonium into nitrites and nitrates.
Its presence links decomposition processes with nutrient cycling and plant productivity.
In aquatic systems, denitrification is often more significant due to widespread anaerobic conditions in sediments.
Algal uptake dominates the assimilation of nitrates and ammonium, linking microbial activity with primary production. Runoff carrying excess nitrates can intensify microbial processes, often leading to eutrophication, which is less common in stable terrestrial ecosystems.
Practice Questions
Question 1 (2 marks)
Identify two processes carried out by microbes that return nitrogen to the atmosphere in the nitrogen cycle.
Mark scheme:
Denitrification (1 mark)
Decomposition leading to ammonium production is not correct here (no mark)
Nitrogen fixation is not correct here (no mark)
Any valid mention of denitrifying bacteria releasing nitrogen gas (N₂) (1 mark)
Maximum: 2 marks
Question 2 (5 marks)
Explain the roles of different types of microbes in maintaining the cycling of nitrogen between organic and inorganic stores.
Mark scheme:
Reference to nitrogen-fixing bacteria (e.g., Rhizobium or Azotobacter) converting N₂ into ammonia (1 mark)
Mention of nitrifying bacteria converting ammonium (NH₄⁺) into nitrite (NO₂⁻) and then nitrate (NO₃⁻) (1 mark)
Identification of denitrifying bacteria converting nitrate (NO₃⁻) back into nitrogen gas (N₂) (1 mark)
Description of decomposer organisms converting organic nitrogen into ammonium (ammonification) (1 mark)
Clear explanation of how these processes ensure availability of nitrogen to plants and maintain ecosystem balance (1 mark)
Maximum: 5 marks

