IB Syllabus focus:
‘Deforestation, agriculture, aquaculture, urbanization alter nitrogen cycling. Haber process boosts yields but drives boundary transgression; coordinated policies and practices are needed to return within planetary limits.’
Human activities have profoundly changed the nitrogen cycle, with synthetic fertilisers, industrial agriculture, and rapid urbanisation altering natural balances and pushing ecosystems beyond safe planetary limits.
Human Impacts on the Nitrogen Cycle
Agricultural Intensification
Large-scale farming relies on nitrogen fertilisers, particularly synthetic fertilisers derived from the Haber process.
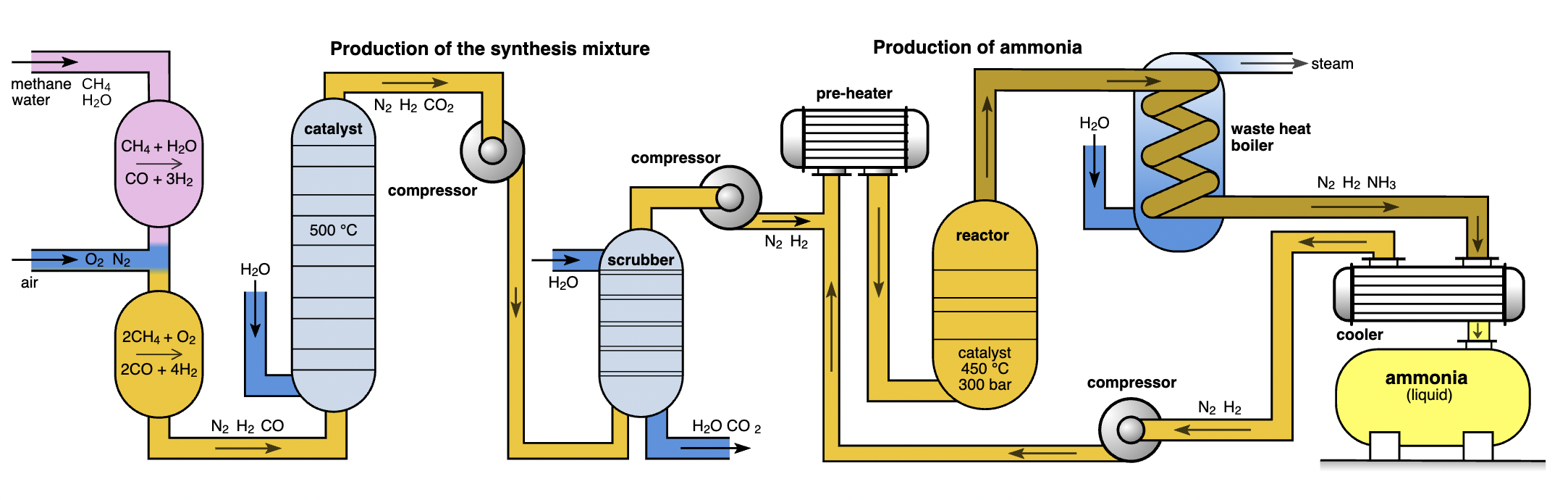
Flow diagram of industrial ammonia synthesis (Haber–Bosch) showing nitrogen and hydrogen feeds, a high-pressure catalytic reactor, cooling/condensation stages, and a recycle loop. This illustrates how industrial fixation scales reactive nitrogen supply for fertilisers, central to human alteration of the nitrogen cycle. Source.
Runoff leads to eutrophication, where excessive nutrients cause algal blooms, oxygen depletion, and aquatic biodiversity loss.

Caption: Landsat-8 image of a harmful algal bloom in Lake Erie (26 September 2017). Such blooms are linked to nutrient enrichment from fertilisers and wastewater, illustrating downstream impacts of anthropogenic nitrogen loading on aquatic systems. Source.
Intensive livestock farming contributes additional nitrogen through manure and waste effluents.
Deforestation and Land Use Change
Deforestation reduces natural nitrogen uptake by plants and disrupts cycling in soils.
Land clearing exposes soils to erosion, increasing nitrogen leaching into rivers and lakes.
Loss of root-associated nitrogen-fixing bacteria diminishes natural ecosystem regulation.
Aquaculture and Urbanisation
Aquaculture systems release nitrogen-rich feed and waste into surrounding waters.
Urbanisation increases sewage, industrial waste, and stormwater runoff, all of which contain high nitrogen loads.
Urban expansion also seals soil surfaces with concrete and asphalt, reducing infiltration and natural cycling.
The Haber Process and Its Consequences
Haber Process: An industrial method combining nitrogen gas (N₂) from the air with hydrogen (H₂) under high temperature and pressure, producing ammonia (NH₃), used in fertilisers.
The process revolutionised agriculture by greatly increasing crop yields and supporting population growth.
However, widespread application has released vast amounts of reactive nitrogen into ecosystems.
Excess ammonia and nitrates contribute to pollution, greenhouse gas emissions (nitrous oxide, N₂O), and biodiversity decline.
Planetary Boundary for Nitrogen
Planetary Boundary: A scientifically defined limit within which humanity can operate safely to avoid destabilising Earth system processes.
The planetary boundary for nitrogen has already been exceeded.
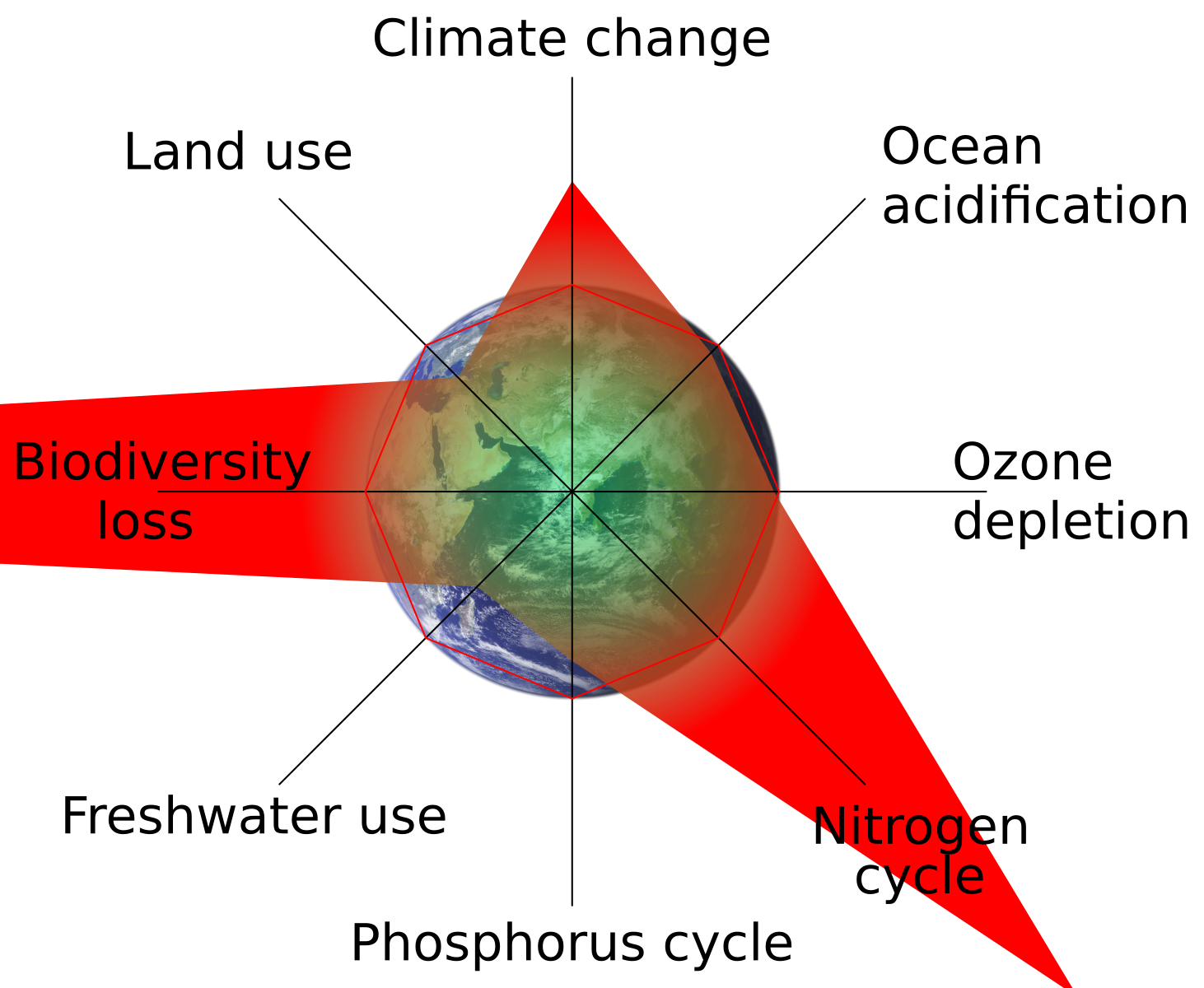
Radial diagram of the planetary boundaries framework; the biogeochemical flows wedge (nitrogen and phosphorus) extends beyond the safe operating space, indicating transgression. This provides a systems context for human-driven nitrogen loading beyond safe global limits; other boundaries shown are additional context not required by the subsubtopic. Source.
Natural nitrogen fixation occurs at around 100–150 million tonnes annually, but human activity has doubled this rate.
Crossing this boundary increases risks of soil degradation, freshwater contamination, and loss of biosphere integrity.
Solutions and Management Strategies
Policy and Regulation
Implement international agreements limiting nitrogen emissions from agriculture and industry.
Establish nutrient management regulations, requiring farmers to monitor and reduce fertiliser use.
Promote environmental taxes on excessive fertiliser application.
Agricultural Practices
Adopt precision farming techniques, applying fertilisers only where and when needed.
Rotate crops with leguminous plants, which host nitrogen-fixing bacteria.
Use organic fertilisers (compost, manure) to recycle nutrients.
Technological and Engineering Solutions
Improve wastewater treatment plants to remove nitrogen compounds before discharge.
Develop slow-release fertilisers that reduce leaching into water bodies.
Explore engineered sequestration methods to capture and store reactive nitrogen.
Ecosystem-Based Approaches
Restore wetlands that naturally filter and store nitrogen.
Reforest degraded land to stabilise soils and enhance nutrient cycling.
Encourage regenerative agriculture that enhances soil organic matter and microbial communities.
Linking Human Nitrogen Use to Sustainability
Excess nitrogen threatens biodiversity, water quality, and climate stability.
Achieving sustainability requires balancing agricultural productivity with ecosystem health.
Coordinated global efforts are essential to bring nitrogen use back within planetary boundaries.
FAQ
Apart from producing fertilisers, the Haber process is highly energy-intensive, relying largely on fossil fuels. This releases significant amounts of carbon dioxide during production.
Additionally, fertiliser use leads to emissions of nitrous oxide, a greenhouse gas with a much greater warming potential than carbon dioxide, further linking the Haber process to climate change.
Carbon dioxide has a single global atmospheric pool, making emissions easier to monitor and regulate.
In contrast, nitrogen pollution occurs in multiple forms—nitrates, nitrites, ammonia, nitrous oxide—and moves through air, water, and soil. This dispersal makes regulation more complex and often requires localised management strategies.
The impacts are also highly regional: fertiliser runoff affects specific watersheds, while nitrous oxide affects the atmosphere globally.
This complexity means thresholds are less precise, making it difficult to set a single safe limit for all ecosystems.
Wetlands act as natural nitrogen sinks by filtering runoff water before it reaches rivers and lakes.
Key processes include:
Microbial denitrification, converting nitrates into nitrogen gas.
Sedimentation, trapping nitrogen in plant matter and soils.
Restoring wetlands is therefore considered one of the most cost-effective strategies for nitrogen management.
Fish farms release uneaten feed and fish waste, both rich in nitrogen compounds.
In coastal waters, this promotes algal blooms, which can lead to hypoxic conditions and fish kills.
Closed or recirculating aquaculture systems can mitigate these impacts, but they are expensive and less common in large-scale operations.
Practice Questions
State one way in which the Haber process has altered the global nitrogen cycle and explain one consequence of this alteration.
Mark scheme:
1 mark for identifying a way the Haber process alters the nitrogen cycle (e.g., industrial fixation of atmospheric N₂, increased fertiliser use).
1 mark for a valid consequence (e.g., eutrophication, biodiversity loss, nitrous oxide emissions).
Question 2 (5 marks)
Discuss how human activities have pushed nitrogen use beyond the planetary boundary, and outline two strategies that could help reduce this transgression.
Mark scheme:
Up to 2 marks for describing how human activities push nitrogen use beyond the boundary (e.g., fertiliser overuse, intensive livestock farming, urbanisation, aquaculture).
1 mark for explicitly linking these activities to the planetary boundary concept.
Up to 2 marks for outlining two appropriate strategies (e.g., precision farming, crop rotation with legumes, wetland restoration, wastewater treatment). Each valid strategy with some explanation earns 1 mark.

