IB Syllabus focus:
‘Pollution occurs when inputs exceed natural transformation. Biodegradability and pollutant half-lives determine persistence and risk.’
Pollution, biodegradability, and half-lives are central to understanding how human activities interact with ecosystems. These concepts highlight the persistence, risks, and management challenges of pollutants worldwide.
Pollution
Pollution arises when inputs of substances or energy into the environment exceed the ability of natural systems to transform, absorb, or neutralise them. This imbalance results in environmental degradation and harm to organisms.
Pollution: The addition of a substance or form of energy to the environment at a rate greater than it can be rendered harmless.
Pollutants can be classified in several ways:
By origin:
Point source (e.g., discharge from a factory).
Non-point source (e.g., agricultural run-off).
By type:
Organic (pesticides, sewage).
Inorganic (heavy metals, plastics).
Energy (heat, noise, radiation).
By duration:
Acute (sudden release, e.g., oil spill).
Chronic (long-term exposure, e.g., pesticides in soils).
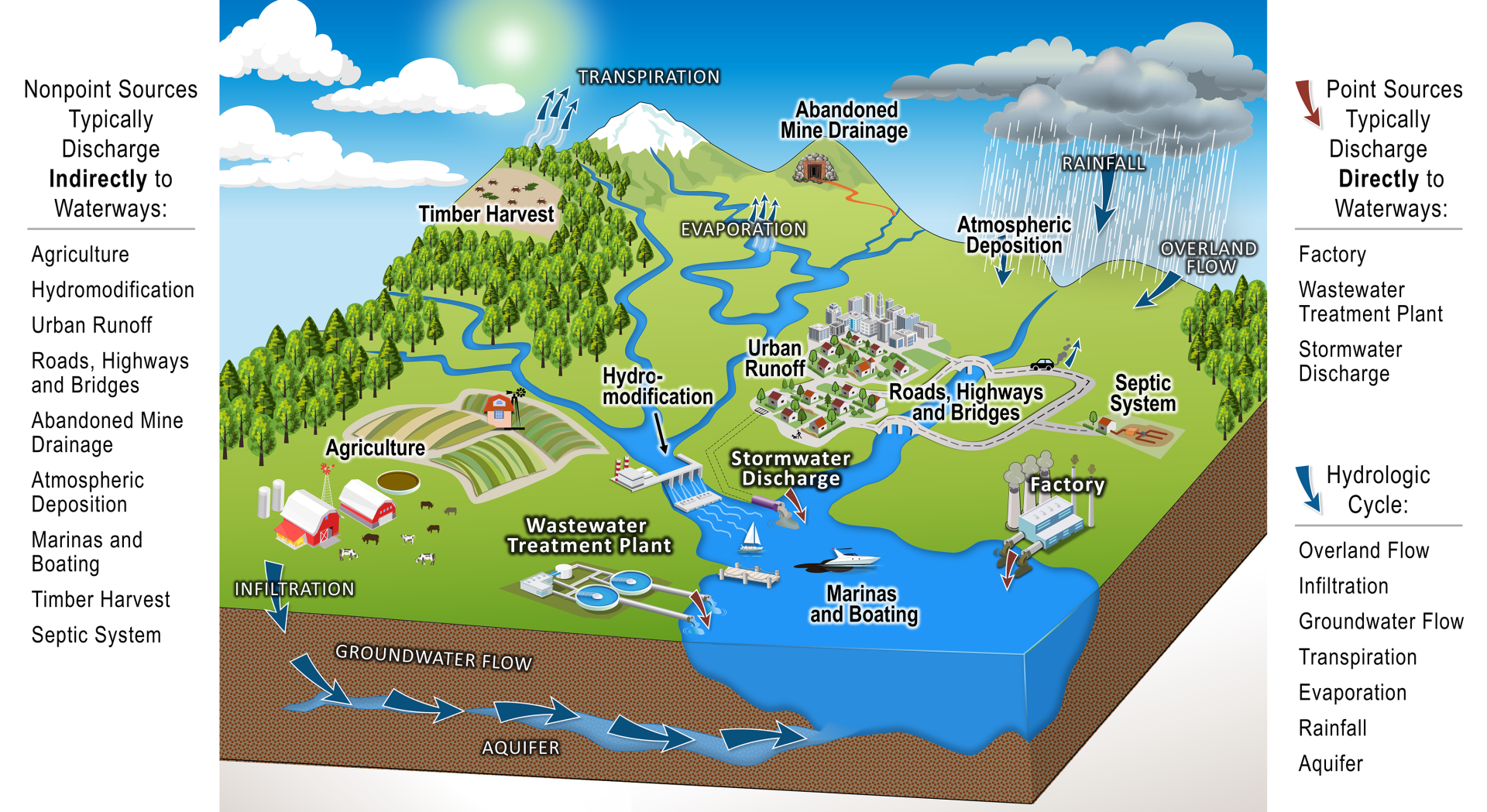
A labelled watershed diagram contrasts point sources (discrete discharges) with non-point sources (diffuse runoff), directly reflecting the classification used in the notes. It also indicates hydrologic pathways that move pollutants into water bodies; this additional hydrology detail exceeds the syllabus but clarifies transport. Source.
The severity of pollution depends on concentration, toxicity, and persistence of the substance involved.
Biodegradability
Biodegradability refers to the ability of a substance to be broken down by natural biological processes, mainly by microorganisms such as bacteria and fungi.
Biodegradability: The capacity of a substance to be decomposed by natural organisms into simpler, non-toxic compounds.
Substances that are highly biodegradable (such as food waste or paper) break down quickly and pose lower long-term risks. Conversely, substances with low biodegradability (such as plastics or polychlorinated biphenyls [PCBs]) can persist in ecosystems for decades.
Biodegradability affects:
Accumulation: Non-biodegradable materials can accumulate in soils, water bodies, and organisms.
Bioaccumulation: The build-up of toxins in an individual organism over time.
Biomagnification: The increase in toxin concentration as it moves up a food chain.
These processes create severe ecological and health risks, especially for persistent organic pollutants (POPs).
Half-lives of Pollutants
The half-life of a pollutant measures the time required for its concentration to decrease by half through natural processes such as degradation, chemical transformation, or radioactive decay.
Half-life: The time taken for the concentration of a pollutant in the environment to reduce by 50%.
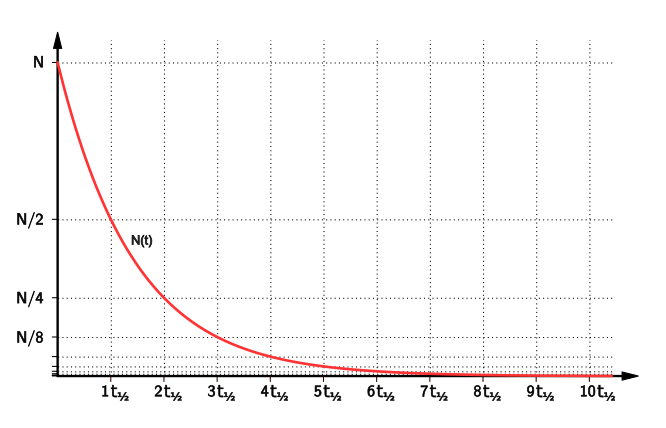
This diagram shows an exponential-decay curve with successive half-life intervals, each halving the remaining quantity. It illustrates why pollutants with longer half-lives persist and pose greater long-term risk. Source.
Pollutants with short half-lives (e.g., many fertilisers) break down quickly, lowering their long-term environmental impacts. In contrast, pollutants with long half-lives (e.g., radioactive isotopes like plutonium or persistent pesticides like DDT) remain hazardous for extended periods.
The half-life influences:
Persistence: How long pollutants remain in ecosystems.
Risk assessment: Long half-life pollutants require stricter regulation.
Management strategies: Disposal and remediation depend on persistence.
Interactions Between Pollution, Biodegradability, and Half-lives
These three factors combine to determine the environmental risk profile of a pollutant:
High biodegradability + short half-life: Low persistence and risk (e.g., organic kitchen waste).
Low biodegradability + long half-life: High persistence and risk (e.g., plastics, heavy metals, radioactive isotopes).
For example:
Plastics are non-biodegradable with effectively infinite half-lives, causing widespread environmental accumulation.
Oil spills contain some biodegradable components, but others (like tar) persist far longer, complicating clean-up efforts.
Radioactive waste, with extremely long half-lives, poses intergenerational risks.
Factors Affecting Biodegradability and Half-lives
Several variables influence how quickly pollutants are broken down:
Environmental conditions: Temperature, oxygen levels, sunlight, and moisture affect degradation rates.
Chemical structure: Complex or synthetic compounds often resist microbial attack.
Ecosystem type: Aquatic, terrestrial, and atmospheric systems vary in their capacity to transform pollutants.
Pollutants in cold, anaerobic, or nutrient-poor environments tend to degrade more slowly, extending their effective half-lives.
Ecological and Societal Implications
The persistence of pollutants has significant implications:
Ecosystem health: Persistent pollutants reduce biodiversity, alter food chains, and damage habitats.
Human health: Non-biodegradable toxins can enter food systems, causing long-term illnesses.
Socioeconomic effects: Pollution clean-up is costly; persistent waste undermines resource security and sustainability.
Communities often face environmental justice concerns, as vulnerable groups may be disproportionately exposed to pollutants with long half-lives and low biodegradability.
Managing Pollution Risks
Effective strategies to address pollution must account for biodegradability and half-lives:
Prevention: Limiting pollutant release before environmental entry.
Substitution: Replacing persistent substances with biodegradable alternatives.
Bioremediation: Using microorganisms to accelerate the breakdown of pollutants.
Legislation and regulation: Controlling emissions, banning certain persistent pollutants (e.g., POPs under the Stockholm Convention).
Education and awareness: Promoting sustainable consumption and waste management.
By understanding these interactions, societies can better balance human needs with long-term environmental sustainability.
FAQ
Non-biodegradable pollutants often have complex molecular structures or synthetic chemical bonds that microorganisms cannot easily break down.
Examples include plastics with strong carbon–carbon bonds, or persistent organic pollutants (POPs) like PCBs and DDT. These compounds resist enzymatic attack and remain in the environment for decades.
Environmental conditions such as low oxygen, low temperature, or limited microbial activity can further slow down any breakdown, enhancing persistence.
Pollutants with long half-lives remain in ecosystems for extended periods, increasing the chances of bioaccumulation and biomagnification.
This persistence means they can travel across food chains and impact top predators, including humans. The intergenerational risks of radioactive isotopes, for example, highlight why long half-life pollutants are particularly hazardous.
For radioactive materials, half-life is fixed and predictable, based on nuclear decay.
In contrast, the half-life of chemical pollutants depends on environmental factors such as sunlight exposure, microbial activity, and oxygen levels. This makes chemical half-lives more variable and context-dependent.
Yes, human interventions can accelerate biodegradation. Methods include:
Adding specific microorganisms (bioremediation).
Adjusting temperature, oxygen, or nutrient levels to optimise microbial activity.
Designing biodegradable materials with chemical structures that break down more readily in natural environments.
Policymakers use half-life data to assess long-term risks and regulate pollutant use.
Pollutants with very long half-lives, such as POPs and radioactive waste, are often banned, restricted, or subject to strict disposal regulations. Shorter half-life pollutants may face less stringent controls but still require monitoring to avoid ecosystem overloading.
Practice Questions
Question 1 (2 marks)
Define the term half-life in the context of environmental pollutants.
Mark Scheme
1 mark for identifying that half-life is the time taken for the concentration of a pollutant to reduce by half.
1 mark for stating that this reduction occurs due to natural processes (e.g., degradation, transformation, or decay).
Question 2 (5 marks)
Discuss how the biodegradability and half-life of pollutants influence their environmental impact. Use examples to support your answer.
Mark Scheme
1 mark for explaining biodegradability as the breakdown of substances by natural organisms.
1 mark for explaining that low biodegradability leads to persistence and accumulation in ecosystems.
1 mark for defining half-life as the time required for a pollutant to decrease in concentration by half.
1 mark for linking long half-life pollutants (e.g., radioactive isotopes, DDT) to higher environmental risk.
1 mark for an example of a pollutant with high biodegradability and short half-life (e.g., organic kitchen waste) contrasted with one of low biodegradability and long half-life (e.g., plastics).

