OCR Specification focus:
‘Describe artificial embryo twinning and somatic cell nuclear transfer, and evaluate uses and issues such as longevity in cloned animals.’
Artificial animal cloning enables scientists to produce genetically identical organisms for research, agriculture, and conservation. It involves manipulating embryos or nuclei to replicate desirable genetic traits precisely.
Artificial Animal Cloning
Artificial cloning in animals replicates natural cloning processes using controlled laboratory methods. It allows the creation of genetically identical individuals without sexual reproduction, using techniques such as artificial embryo twinning and somatic cell nuclear transfer (SCNT). These techniques have significant applications in agriculture, medicine, and species preservation, but also raise ethical and biological concerns.
Artificial Embryo Twinning
Artificial embryo twinning mimics the natural process of embryo splitting that produces identical twins. This method is an early, simple form of cloning used mainly in livestock breeding and research.
Process
A fertilised egg (zygote) is produced via in vitro fertilisation (IVF).
The early-stage embryo (usually at the blastomere or morula stage) is carefully split into two or more cells.
Each separated cell is allowed to develop into an independent embryo in a suitable culture medium.
These cloned embryos are then implanted into surrogate mothers, producing genetically identical offspring.
This technique is limited to early embryonic stages and relies on totipotent cells capable of forming all body tissues. It is particularly valuable in producing multiple copies of animals with desirable genetic traits, such as high milk yield in cattle or disease resistance.
Somatic Cell Nuclear Transfer (SCNT)
Somatic Cell Nuclear Transfer (SCNT) is a more advanced and precise cloning method that can create a clone of an existing individual rather than a newly fertilised embryo.
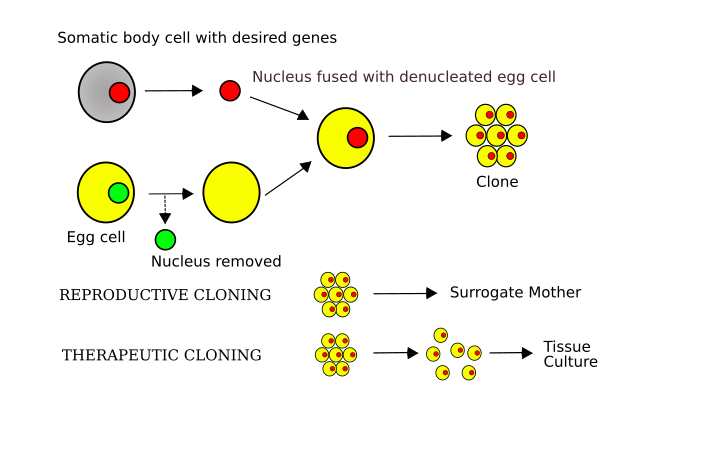
Diagram of somatic cell nuclear transfer: the nucleus from a donor somatic cell is transferred into an enucleated egg, which is activated to divide. The embryo can be implanted for reproductive cloning. Note: the right-hand branch labelled ‘therapeutic cloning’ is extra detail beyond the OCR subsubtopic. Source.
Somatic Cell Nuclear Transfer: A cloning technique where the nucleus of a somatic (body) cell is transferred into an enucleated egg cell to produce a genetically identical organism.
Process
A somatic cell (e.g., from skin or udder tissue) is collected from the donor animal.
An unfertilised egg cell is taken from another animal of the same species.
The nucleus is removed from the egg cell, leaving an enucleated ovum.
The donor nucleus is inserted into the enucleated egg, either by microinjection or electrofusion (using an electric current to fuse membranes).
The reconstructed cell is stimulated to divide as if it were a fertilised egg.
The developing embryo is cultured until it reaches the blastocyst stage.
Finally, it is implanted into a surrogate mother to develop into a live clone.
The most famous example of SCNT is Dolly the sheep, cloned in 1996 from the nucleus of an adult mammary cell. Dolly demonstrated that specialised adult cells could be reprogrammed to form a complete organism.

Dolly the sheep on display at the National Museum of Scotland. Dolly was the first mammal cloned from an adult somatic cell, exemplifying the feasibility of reprogramming differentiated nuclei. The photo supports evaluation points on efficiency and longevity in cloned animals. Source.
Applications of Artificial Cloning
Artificial cloning techniques have diverse scientific and commercial uses:
Agricultural improvement – Farmers can reproduce livestock with superior genetics, such as higher milk production or leaner meat.
Conservation biology – Cloning endangered or even recently extinct species may help preserve biodiversity when genetic material is available.
Biomedical research – Genetically uniform animals are used in studies of disease, drug testing, and gene expression to reduce variability in results.
Therapeutic cloning – Although ethically controversial, cloning embryos for stem cell extraction can provide tissues genetically identical to a patient, potentially reducing transplant rejection.
Reproductive cloning – Used to replicate elite breeding animals or genetically engineered specimens, ensuring consistent performance in research or agriculture.
Evaluation of Artificial Animal Cloning
Cloning raises complex scientific, ethical, and biological questions. Evaluation involves balancing potential benefits against limitations and moral considerations.
Advantages
Genetic uniformity: Ensures desired traits are preserved and replicated consistently.
Control over reproduction: Allows reproduction without relying on unpredictable sexual reproduction.
Research benefits: Facilitates the study of genetic diseases, developmental biology, and gene regulation in identical genetic contexts.
Biodiversity preservation: Offers potential to rescue endangered species through somatic cell storage and reprogramming.
Therapeutic potential: Enables development of genetically matched tissues and organs for regenerative medicine.
Disadvantages and Issues
Reduced genetic diversity: Clones are genetically identical, making populations more vulnerable to diseases or environmental changes.
Low success rates: Many embryos fail to develop properly; success rates can be as low as 1–2%.
Developmental abnormalities: Clones may suffer from immune deficiencies, organ failure, or premature ageing due to incomplete nuclear reprogramming.
Longevity concerns: Studies, such as those involving Dolly, suggest clones may have shorter lifespans, though later evidence indicates this varies.
Ethical concerns: Questions arise about animal welfare, the moral justification for cloning, and potential future use in humans.
High costs: Cloning is expensive, labour-intensive, and not commercially viable for large-scale farming.
Longevity and Genetic Stability
A key issue with SCNT is epigenetic reprogramming. The transferred nucleus must revert from a specialised to a totipotent state, but this process is often incomplete.
Epigenetic Reprogramming: The resetting of chemical modifications (epigenetic marks) on DNA and histones that control gene expression without altering the DNA sequence.
Incomplete reprogramming can cause abnormal gene expression, resulting in developmental defects or early ageing symptoms. Although Dolly lived to six years (half the normal lifespan of a sheep), later studies suggested her arthritis and death were not directly due to cloning, highlighting the complexity of longevity assessment.
Ethical and Regulatory Perspectives
Artificial cloning provokes debate between utilitarian perspectives (maximising benefits for humans and animals) and deontological ethics (questioning the moral rightness of creating life artificially).
Key ethical issues include:
The instrumental use of animals for human benefit.
Suffering caused by high embryo failure and abnormalities.
The slippery slope toward human reproductive cloning.
Ownership and patenting of cloned organisms.
Regulations vary internationally: in the UK, animal cloning is regulated by the Human Fertilisation and Embryology Authority (HFEA) and the Animals (Scientific Procedures) Act, which restrict cloning to scientific or therapeutic purposes under strict ethical review.
Future Directions
Advances in cloning are improving success rates through better understanding of gene expression, epigenetics, and stem cell reprogramming. Researchers aim to refine SCNT and related technologies such as induced pluripotent stem cells (iPSCs) to reduce ethical concerns and biological risks, while maintaining scientific and agricultural benefits.
FAQ
Success rates vary widely between species due to differences in egg cell quality, reprogramming efficiency, and developmental compatibility.
In sheep and cattle, success rates are typically below 5%, meaning only a few viable offspring per hundred embryos.
In pigs and horses, rates can be even lower due to more complex oocyte structures and sensitivity to manipulation.
Mice and some small mammals often show higher success in experimental conditions because of greater control over embryo culture and implantation.
These differences highlight that cloning remains species-dependent and technically challenging.
Cloned animals often display Large Offspring Syndrome (LOS) or developmental abnormalities due to irregular gene expression.
During reprogramming, epigenetic marks controlling genes related to growth and metabolism may not reset correctly. This leads to abnormal hormone levels such as insulin-like growth factors (IGFs).
Overexpression of growth-related genes can cause unusually large fetuses and birthing complications.
Underexpression may produce smaller or weaker clones.
Improving nuclear reprogramming techniques reduces these inconsistencies.
The most suitable donor cells are somatic cells with stable, intact nuclei and minimal genetic damage.
Common examples include:
Fibroblasts (from skin tissue) – easy to culture and control.
Mammary gland cells – used famously for cloning Dolly the sheep.
Cumulus cells – often used in cattle cloning due to their active state during oogenesis.
Younger donor cells are generally preferred as they contain fewer DNA mutations and shorter exposure to environmental stress.
Genetic verification involves molecular and phenotypic testing to confirm identity.
DNA profiling using microsatellites or short tandem repeats (STRs) confirms identical genetic markers.
Mitochondrial DNA (from the egg donor) is checked to confirm it differs from the nuclear donor, as mitochondria are maternally inherited.
Physical traits and biochemical markers are observed, but genetic tests remain the definitive confirmation.
This process ensures the clone is a true nuclear replica rather than a similar relative.
Cloning offers a way to preserve the genetics of endangered or recently extinct species when breeding populations are too small.
Somatic cells from preserved tissue can be used to recreate individuals.
Hybrid surrogates (closely related species) may carry cloned embryos when the original species’ surrogates are unavailable.
It allows the reintroduction of lost genetic diversity by reviving rare genotypes.
However, cloning alone cannot sustain populations; conservation must also address habitat protection and natural breeding restoration.
Practice Questions
Question 1 (2 marks)
State what is meant by somatic cell nuclear transfer (SCNT) and name one animal that was successfully cloned using this technique.
Mark scheme:
1 mark: Correctly defines SCNT as the process in which the nucleus of a somatic (body) cell is transferred into an enucleated egg cell.
1 mark: Names Dolly the sheep (or any other correctly identified cloned animal such as Snuppy the dog or CC the cat).
Question 2 (5 marks)
Describe the main steps involved in producing a cloned animal by somatic cell nuclear transfer and discuss one potential advantage and one potential disadvantage of this technique.
Mark scheme:
1 mark: States that a somatic cell is taken from the donor animal.
1 mark: States that an egg cell is enucleated (its nucleus is removed).
1 mark: Describes that the donor nucleus is inserted into the enucleated egg (by microinjection or electrofusion).
1 mark: Mentions that the embryo develops and is implanted into a surrogate mother.
1 mark: Gives one clear advantage (e.g. production of genetically identical animals with desirable traits, preservation of endangered species) and one clear disadvantage (e.g. low success rate, reduced genetic diversity, or ethical concerns).

