OCR Specification focus:
‘Explain why microorganisms are widely used in biotechnological processes, considering growth requirements, life cycles and economic factors.’
Microorganisms play a vital role in biotechnology due to their rapid growth, diverse metabolic capabilities, and cost-effectiveness, enabling efficient production of valuable substances for medicine, agriculture and industry.
The Role of Microorganisms in Biotechnology
Microorganisms such as bacteria, fungi, and yeasts are indispensable to biotechnology. They serve as biological factories that transform raw materials into useful products through biochemical processes like fermentation, enzyme synthesis, and bioconversion. Their use spans pharmaceuticals, food production, waste treatment, and biofuel development.
Why Microorganisms Are Widely Used
1. Rapid Growth and Simple Nutritional Requirements
Microorganisms reproduce rapidly, often doubling their population within hours. Their short generation time allows industries to achieve high yields in short periods.
They require relatively simple growth media, containing sources of carbon, nitrogen, and essential minerals, which keeps production costs low.
Generation Time: The time taken for a population of microorganisms to double in number under optimal conditions.
Microorganisms can grow on inexpensive substrates, including agricultural waste or by-products from other industries, enhancing sustainability and reducing the cost of raw materials.
2. Versatile Metabolic Pathways
Microorganisms have extremely diverse metabolic capabilities, enabling them to carry out numerous biochemical transformations. These include:
Fermentation: Conversion of sugars into alcohol, acids, or gases under anaerobic conditions.
Aerobic respiration: Production of biomass and secondary metabolites such as antibiotics.
Biotransformation: Chemical modification of compounds, such as converting steroids into therapeutic forms.
Because of this versatility, microorganisms can synthesise complex molecules that are difficult or expensive to produce chemically.
3. Genetic Manipulation and Control
Microorganisms are ideal for genetic engineering, as their genomes are relatively small and well understood. Recombinant DNA technology enables scientists to introduce genes for desired products. For example:
Escherichia coli can be modified to produce human insulin.
Saccharomyces cerevisiae (yeast) can express vaccine antigens.
Bacillus subtilis can produce enzymes used in detergents.
Their ease of modification allows precise control over product characteristics and yields, revolutionising modern biotechnology.
Growth Requirements for Microorganisms in Biotechnological Processes
The effectiveness of microbial biotechnology depends on providing optimal growth conditions. Key requirements include:
1. Nutrient Supply
Microorganisms need macronutrients and micronutrients to sustain growth.
Carbon source: usually glucose, providing energy and building blocks.
Nitrogen source: ammonium salts or nitrates for amino acid synthesis.
Minerals: magnesium, iron, and potassium for enzyme activation and structural roles.
2. Environmental Conditions
Temperature: Most industrial microbes are mesophilic, growing best between 20–40 °C.
pH: Enzyme activity and membrane transport depend on optimal pH, often near neutral (pH 6–8).
Oxygen availability: Aerobic and anaerobic organisms require specific aeration conditions to achieve maximal productivity.
Water availability: Essential for metabolic reactions and nutrient transport.
Bioprocess: A controlled microbial or enzymatic process used to produce desired biological products under optimised environmental conditions.
Modern biotechnological systems use bioreactors with automated monitoring of these parameters to ensure consistent yields and prevent contamination.
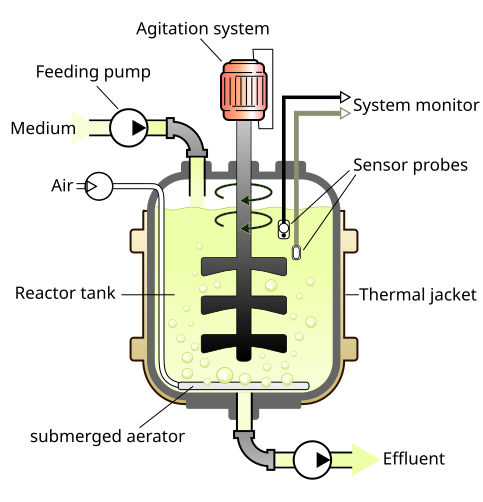
Stirred-tank bioreactor schematic illustrating the vessel, impeller, gas inlet/sparging, sampling/ports, and temperature jacket used for control. Such systems provide the regulated oxygen transfer, mixing, and thermal management required for high, consistent yields in industrial microbiology. Diagram elements mirror the parameters discussed in the notes; minor iconography beyond the OCR scope is limited to basic vessel labelling. Source.
Microbial Life Cycles and Their Biotechnological Importance
The life cycle of microorganisms determines the timing of product formation and harvesting.
1. Growth Phases in Batch Culture
Microbial growth follows a standard pattern known as a growth curve, consisting of four main phases:
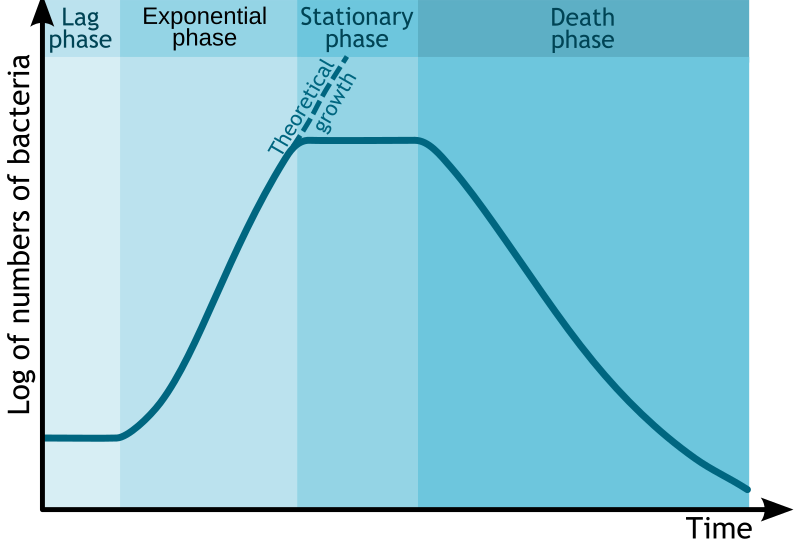
Bacterial growth curve showing lag, exponential (log), stationary, and death phases with cell number on a log scale against time. This figure helps link growth phase to industrial decisions about when to harvest biomass or secondary metabolites. The layout is clean and labels align with OCR terminology; no extraneous content is included. Source.
Lag phase: Adjustment to new conditions; metabolic activity increases but no cell division yet.
Log (exponential) phase: Rapid cell division; product yield and enzyme synthesis are high.
Stationary phase: Nutrients become limited; secondary metabolites such as antibiotics are produced.
Death phase: Toxic waste accumulates; cell numbers decline.
Secondary Metabolite: A compound produced by microorganisms not essential for growth but often with ecological or industrial value, such as antibiotics.
2. Process Selection
Batch culture: All nutrients provided at the start; ideal for secondary metabolites.
Continuous culture: Nutrients constantly supplied and waste removed; supports constant growth and steady product output.
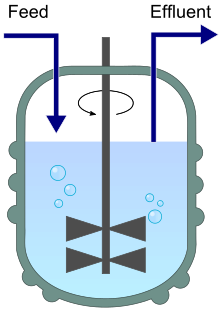
Chemostat diagram indicating continuous medium inflow and culture outflow that keep volume constant while fixing the dilution rate. This arrangement maintains cells at a defined physiological steady state for stable product formation — the key advantage noted in the syllabus focus on growth requirements and life cycles. The figure is intentionally minimalist and avoids extraneous detail. Source.
The choice of process depends on whether the desired product is a primary or secondary metabolite.
Economic Factors in Microbial Biotechnology
Microbial processes are economically attractive compared with traditional chemical synthesis due to their efficiency and scalability.
1. Cost Efficiency
Microbes thrive on inexpensive substrates, including waste materials.
Production occurs at moderate temperatures and pressures, reducing energy expenditure.
Minimal labour requirements once systems are automated.
2. High Yield and Product Purity
Microbial cultures can be maintained under sterile conditions, minimising contamination.
Products such as enzymes, antibiotics, and amino acids can be harvested in high concentrations.
Use of immobilised cells or enzymes enables repeated use, reducing costs further.
3. Sustainability and Environmental Benefits
Microbial biotechnology supports green chemistry principles by replacing polluting chemical reactions with biodegradable, low-energy biological alternatives. Examples include:
Bioremediation using bacteria to degrade pollutants.
Biofuel production from yeast and algae.
Composting driven by microbial decomposition of organic waste.
These sustainable technologies make microbial biotechnology a cornerstone of the circular economy.
Industrial and Commercial Applications
1. Pharmaceuticals
Penicillium chrysogenum produces penicillin, an antibiotic.
Streptomyces species produce other valuable antibiotics and immunosuppressants.
2. Food and Drink
Saccharomyces cerevisiae ferments sugar to produce ethanol in beer and wine.
Lactobacillus species ferment milk to produce yoghurt and cheese.
3. Enzyme Production
Microbial enzymes such as amylase, protease, and lipase are vital in industries including detergents, textiles, and pharmaceuticals.
Biotechnologists favour microbes for enzyme production because they can secrete large quantities directly into the culture medium.
4. Waste Treatment
Bacteria decompose organic waste in sewage treatment plants, while specialised microbes detoxify industrial effluents.
Advantages and Considerations
Advantages
Fast growth and reproduction rates.
Ease of genetic modification.
Consistent and predictable production.
Wide range of potential products.
Environmentally sustainable methods.
Limitations
Risk of contamination by unwanted microorganisms.
Ethical and safety considerations for genetically modified strains.
Requirement for sterile and controlled environments.
FAQ
Microorganisms are preferred because they are easier and faster to grow, requiring less space and fewer resources than plants or animals. Their rapid reproduction allows continuous or large-scale production within controlled environments such as bioreactors.
They can also be genetically modified with precision, enabling the production of specific enzymes, metabolites, or pharmaceuticals. Unlike higher organisms, microbes are not limited by ethical concerns, complex life cycles, or long growth times.
The choice depends on the product being made and the organism’s metabolic requirements.
Aerobic processes (with oxygen) are used when oxygen is needed for energy generation and product synthesis, such as enzyme or biomass production.
Anaerobic processes (without oxygen) are used for fermentation products like ethanol or lactic acid.
Oxygen availability, energy yield, and desired product type all influence which metabolic pathway is preferred.
Microorganisms support sustainability by replacing chemical synthesis with biological processes that use renewable materials and produce less waste.
They grow on low-cost substrates like agricultural waste or glycerol by-products.
Many microbial processes operate at moderate temperatures and pressures, reducing energy consumption.
Microbes also play a role in waste management through bioremediation, converting pollutants into harmless substances.
Overall, microbial biotechnology aligns with circular economy principles and reduces industrial carbon footprints.
Safety protocols are vital to prevent contamination and protect workers and the environment.
Work is carried out in aseptic conditions to prevent cross-contamination.
Microorganisms are categorised into biosafety levels (BSL 1–4) depending on their pathogenicity.
Proper sterilisation of equipment and waste using autoclaves or disinfectants ensures no live microbes are released.
Risk assessments and regulatory guidelines govern the use of genetically modified strains.
An ideal microorganism for industrial fermentation should possess:
Fast growth rate and ability to thrive under simple, inexpensive conditions.
Genetic stability, ensuring consistent product yield over multiple generations.
Non-pathogenicity, posing no health risks to humans.
High product yield and easy product extraction.
Tolerance to fluctuations in temperature, pH, or oxygen levels.
These traits ensure efficiency, safety, and profitability in large-scale production systems.
Practice Questions
Question 1 (2 marks)
Explain two reasons why microorganisms are widely used in biotechnological processes.
Mark Scheme
Microorganisms have rapid growth rates and short generation times, allowing products to be formed quickly. (1 mark)
They have simple nutritional requirements, often growing on inexpensive substrates or waste materials, reducing production costs. (1 mark)
Accept alternative valid points such as: easily genetically modified; wide range of metabolic pathways.
Question 2 (5 marks)
Describe and explain how growth conditions in a bioreactor are controlled to maximise the yield of a desired product from microorganisms.
Mark Scheme
Temperature is monitored and maintained at the microorganism’s optimum to ensure enzymes function efficiently without denaturation. (1 mark)
pH control using buffers or automatic neutralisation maintains enzyme activity and prevents growth inhibition. (1 mark)
Aeration and mixing ensure even distribution of oxygen (for aerobic species) and nutrients throughout the culture. (1 mark)
Nutrient levels are monitored; in continuous culture, fresh medium is added and waste removed to maintain cells in exponential growth. (1 mark)
Sterility is maintained to prevent contamination, which could reduce yield or destroy the culture. (1 mark)
Allow references to sensors, feedback systems, or automatic control systems for precision as valid elaboration.

