OCR Specification focus:
‘Outline methods for immobilising enzymes and evaluate applications, including glucose isomerase, penicillin acylase, lactase, aminoacylase and glucoamylase.’
Immobilised enzymes are central to modern biotechnology, offering enhanced stability, reusability and efficiency in industrial processes that require rapid, cost-effective and environmentally sustainable biochemical reactions.
What Are Immobilised Enzymes?
Immobilised enzymes are enzymes that are attached to or trapped within an insoluble material so that they can be retained and reused in industrial reactions without being lost in the product. This approach improves enzyme stability, reduces operational costs, and allows continuous production.
Immobilised enzyme: An enzyme fixed to, or trapped within, an inert and insoluble support material, allowing reuse and controlled reaction conditions.
Unlike free enzymes, which dissolve in solution and are difficult to separate after use, immobilised enzymes remain localised and can operate over extended periods.
Methods of Immobilisation
Several techniques are used to immobilise enzymes, each with distinct properties affecting enzyme activity and suitability for different industrial applications.

Enzymes immobilised in calcium alginate beads used for entrapment. The porous hydrogel retains the enzyme while allowing substrates and products to diffuse. This real-world view complements the schematic methods by showing the typical appearance of alginate immobilisation beads. Source.
Adsorption
Enzymes adhere to the surface of an inert support such as glass beads, clay, or porous carbon through hydrophobic interactions or ionic bonds.
Advantages: Simple, inexpensive, and retains high enzyme activity.
Disadvantages: Enzyme may detach due to changes in pH, temperature or ionic strength, causing contamination.
Covalent Bonding
Enzymes form covalent bonds with the support material (e.g. cellulose, silica, or synthetic polymers).
Advantages: Strong attachment prevents enzyme leakage; good for continuous processes.
Disadvantages: Covalent linkage may alter enzyme shape, reducing activity.
Entrapment
The enzyme is trapped within a matrix such as gel beads (e.g. alginate or polyacrylamide).
Advantages: Protects enzyme from environmental change; minimal leakage.
Disadvantages: Substrate diffusion is limited; slower reaction rate.
Encapsulation
The enzyme is enclosed within a semipermeable membrane or microcapsule, allowing substrate and product exchange while retaining the enzyme.
Advantages: High protection from denaturation; suitable for small enzymes.
Disadvantages: Diffusion through the membrane may be slow.
Cross-Linking
Enzymes are chemically bonded to each other using a cross-linking agent such as glutaraldehyde, forming large, insoluble aggregates.
Advantages: No carrier required; high stability.
Disadvantages: Possible enzyme distortion; difficult to control.
Advantages of Immobilised Enzymes
Immobilised enzymes provide several benefits that make them ideal for industrial biotechnology:
Reusability: Enzymes can be recovered and reused multiple times, reducing costs.
Stability: Increased resistance to changes in temperature and pH.
Continuous operation: Useful in continuous-flow bioreactors.
Product purity: Enzymes do not mix with the final product, simplifying purification.
Efficiency: Enables higher substrate concentrations and faster reaction rates in optimised systems.
Reduced contamination risk: Fixed enzymes minimise microbial growth in reactors.
However, immobilisation can reduce enzyme activity due to restricted mobility or diffusion limits, which must be managed through careful design of support materials and reaction conditions.
Applications of Immobilised Enzymes in Biotechnology
Immobilised enzymes are integral to the food, pharmaceutical, and chemical industries, where precision and reliability are essential.
Glucose Isomerase
Glucose isomerase converts glucose to fructose, a process used in manufacturing high-fructose corn syrup (HFCS).
The enzyme is immobilised to allow continuous conversion in packed-column reactors.
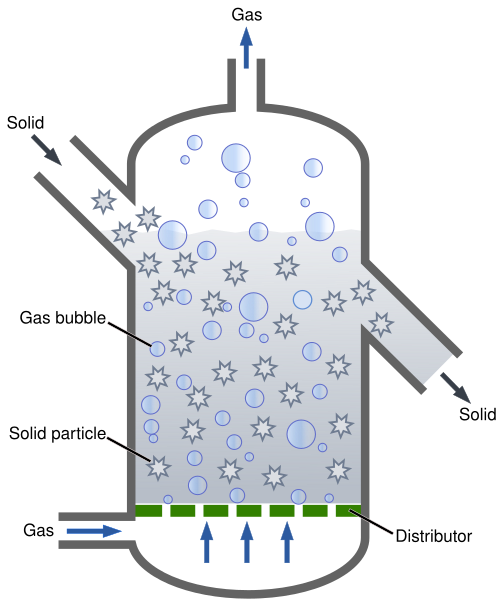
Simplified schematic of a fluidised-bed reactor, where upward flow suspends solid particles (e.g., immobilised enzyme beads) to enhance mixing and mass transfer. While the diagram is general chemical-engineering content, its layout applies directly to continuous immobilised-enzyme bioreactors covered in the notes. Source.
Advantages: Efficient, high-yield process; enzyme stability ensures consistent product quality.
Used in: Soft drinks and confectionery industries.
Penicillin Acylase
Penicillin acylase is used to convert penicillin into 6-aminopenicillanic acid (6-APA), the base for producing semisynthetic antibiotics.
Immobilised penicillin acylase enables continuous antibiotic production.
Advantages: High purity of product, cost-effective, and reduced environmental waste.
Lactase
Lactase (β-galactosidase) hydrolyses lactose into glucose and galactose, producing lactose-free milk.
Immobilised lactase is used in dairy processing columns.
Benefits: Allows continuous production; enzyme remains active over long periods.
Relevance: Important for consumers with lactose intolerance.
Aminoacylase
Aminoacylase separates optical isomers of amino acids, vital in pharmaceutical synthesis where only one isomer is biologically active.
Immobilised aminoacylase ensures stereospecificity and reusability.
Applications: Production of L-amino acids used in medicine and nutrition.
Glucoamylase
Glucoamylase breaks down starch into glucose, commonly used in brewing, baking, and biofuel production.
Immobilised glucoamylase allows continuous starch hydrolysis in industrial fermenters.
Advantages: Stable performance under industrial conditions; easy enzyme recovery.
Factors Affecting Immobilised Enzyme Performance
Several factors influence the effectiveness and longevity of immobilised enzymes:
Support material: Must be inert, porous, and mechanically stable.
Temperature and pH: Immobilisation often increases tolerance, but extremes still reduce activity.
Substrate diffusion: Limited movement within matrices can slow reaction rates.
Enzyme loading: Too high concentration can cause crowding and lower efficiency.
Operational stability: Over time, enzyme may gradually denature or detach.
Optimising these conditions is key to maximising product yield and reducing production costs.
Evaluation of Immobilised Enzyme Use
Advantages:
Enables large-scale, sustainable production.
Reduces environmental waste and resource consumption.
Provides consistent product quality.
Disadvantages:
High initial setup cost for immobilisation systems.
Possible loss of enzyme activity during immobilisation.
Complex optimisation required for maximum yield.
Despite these challenges, immobilised enzyme systems are considered a cornerstone of industrial biotechnology, combining biological specificity with engineering control to meet modern production demands efficiently and sustainably.
FAQ
Immobilisation can slightly alter the enzyme’s tertiary structure, which may affect the shape of the active site. This can reduce substrate affinity and reaction rate.
However, some immobilisation methods, such as entrapment or encapsulation, protect the enzyme from environmental stress, maintaining its activity for longer.
The overall effect depends on the method used and the strength of bonding between the enzyme and the support material — strong covalent bonds can cause more distortion than weaker adsorption methods.
In immobilised systems, substrates must diffuse through a barrier (such as a gel or matrix) to reach the enzyme, and products must diffuse out.
This creates a rate-limiting step, especially when:
The substrate molecules are large or non-polar.
The matrix is thick or densely cross-linked.
The reaction occurs rapidly, but diffusion cannot keep up.
Optimising pore size and flow rate in bioreactors helps reduce diffusion limitations and maintain efficient reaction rates.
An effective support material should have the following characteristics:
Inertness: It must not react chemically with the enzyme or substrate.
Porosity: Allows substrates and products to move freely.
Mechanical strength: Must withstand industrial conditions and fluid flow.
Chemical stability: Should remain stable over the required pH and temperature ranges.
Biocompatibility: Should not denature the enzyme upon contact.
Common materials include silica, alginate, cellulose, and polyacrylamide, chosen based on the desired application and enzyme sensitivity.
Over time, immobilised enzymes lose activity due to denaturation, leaching, or fouling.
In industrial systems:
Reactors are often designed with modular columns or cartridges, allowing easy removal and replacement of enzyme beds.
Chemical regeneration can sometimes restore partial activity if the enzyme has not degraded.
Spent supports may be washed and recoated with fresh enzyme, reducing material waste.
This ensures long-term productivity and minimal downtime in continuous operations.
Yes. Immobilised enzymes are used in biosensors and diagnostic devices for accurate, long-term monitoring of biochemical substances.
Examples include:
Glucose oxidase immobilised on electrodes for blood glucose monitoring.
Urease or cholesterol oxidase immobilised in diagnostic strips.
Immobilisation stabilises the enzyme and prevents contamination, allowing sensors to function reliably for extended periods — a critical feature for clinical and point-of-care use.
Practice Questions
Question 1 (2 marks)
State two advantages of using immobilised enzymes rather than free enzymes in an industrial process.
Mark scheme:
Award 1 mark for each correct advantage, up to a maximum of 2 marks.
Enzymes can be reused, reducing production costs. (1)
Product is not contaminated with enzyme, making purification easier. (1)
Immobilised enzymes are more stable over a range of pH and temperatures. (1)
Allows continuous operation in bioreactors. (1)
Question 2 (5 marks)
Describe and explain two different methods of enzyme immobilisation, and discuss one advantage and one disadvantage of using immobilised enzymes in biotechnology.
Mark scheme:
Award marks as follows:
Description of methods (maximum 3 marks):
Adsorption: Enzyme attaches to the surface of an inert support, such as glass beads or clay, by weak bonds (hydrophobic or ionic). (1)
Covalent bonding: Enzyme forms strong covalent bonds with a support material, preventing enzyme loss. (1)
Entrapment: Enzyme trapped in a matrix such as alginate gel or polyacrylamide beads. (1)
Encapsulation or cross-linking also creditworthy if correctly described. (1)
Explanation (1 mark):
Immobilisation allows enzymes to be reused and stabilises them against temperature and pH changes. (1)
Evaluation (1 mark for each point, maximum 2 marks):
Advantage: Product remains enzyme-free, simplifying purification, or allows continuous production. (1)
Disadvantage: Reduced enzyme activity due to restricted substrate diffusion or altered enzyme shape. (1)
Total = 5 marks.

