OCR Specification focus:
‘Describe aseptic technique, effective culturing, and how batch and continuous fermentation conditions are manipulated to maximise product yield; interpret standard microbial growth curves.’
Microorganisms are invaluable in biotechnology, producing antibiotics, enzymes, and foods. Understanding how to culture microbes effectively and optimise conditions for maximum yield is essential for biotechnological success.
Culturing Microorganisms
Culturing microorganisms involves growing bacteria, fungi, or yeasts under controlled laboratory or industrial conditions. These organisms can produce valuable metabolites such as enzymes, hormones, and antibiotics. Culturing requires careful control of physical and chemical parameters to encourage rapid and safe microbial growth.
Aseptic Technique
Aseptic technique ensures that no unwanted microorganisms contaminate the culture, protecting both the integrity of the experiment and the safety of the operator.
Aseptic Technique: A set of procedures used to prevent contamination of cultures and the environment by unwanted microorganisms.
Key aseptic procedures include:
Sterilisation of equipment using autoclaves at 121°C and high pressure to kill all microbes.
Disinfection of work surfaces before and after use.
Flame sterilisation of inoculating loops before contact with microorganisms.
Minimising exposure by keeping lids partially closed and avoiding unnecessary talking or movement near open cultures.
Use of biological safety cabinets for high-risk organisms.
Aseptic techniques are vital for maintaining pure cultures, allowing accurate study of microbial characteristics or maximising the production of a desired product in industrial fermentation.
Microbial Growth
Microbial growth refers to the increase in the number of cells in a population, not the size of individual cells. Growth can be measured using optical density, cell counts, or dry mass.
Standard Microbial Growth Curve
Microbial growth follows a typical pattern when cultured in a closed system, such as a batch culture:
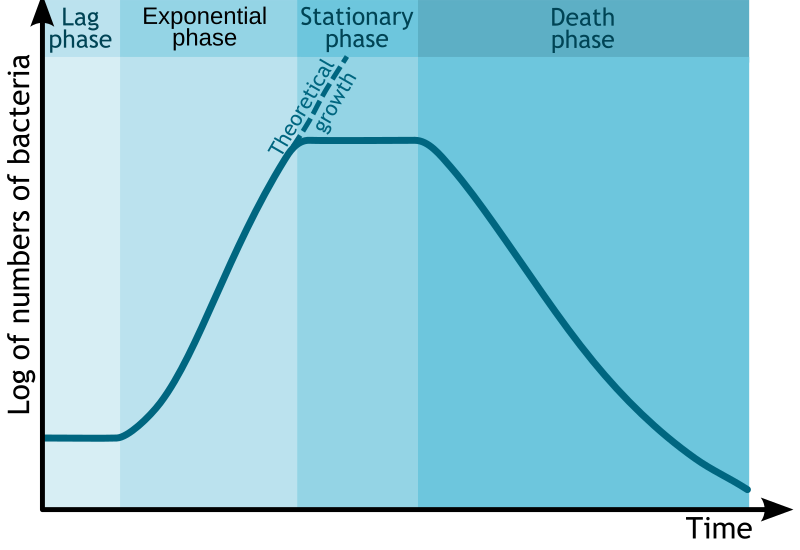
A standard microbial growth curve for a batch culture showing lag, exponential (log), stationary, and death phases. This visual anchors discussion of when to harvest primary versus secondary metabolites. Source.
Lag phase: Microbes adjust to their environment, synthesising enzymes and molecules needed for growth.
Log (exponential) phase: Cells divide rapidly; growth rate is at its maximum.
Stationary phase: Nutrients become limited and waste accumulates; growth rate equals death rate.
Death (decline) phase: Nutrients are depleted, and toxic products build up, causing population decline.
Batch Culture: A closed culture system where microorganisms grow in a fixed volume of medium with no addition or removal of nutrients during the process.
Interpreting microbial growth curves allows scientists to identify optimum conditions for maximum yield and determine when to harvest products — for instance, during the stationary phase for secondary metabolites such as antibiotics.
Optimising Conditions for Growth
Microbial yield and productivity depend on environmental and nutritional conditions. Each parameter must be controlled carefully in bioreactors.
Physical Conditions
Temperature: Influences enzyme activity; too high leads to denaturation, too low slows metabolism.
pH: Each microorganism has an optimum pH for enzyme function. pH is monitored and adjusted using buffers or automatic systems.
Oxygen availability: Aerobic organisms require oxygen for respiration; anaerobic conditions promote fermentation.
Agitation: Stirring ensures uniform distribution of nutrients and oxygen throughout the culture.
Nutritional Conditions
Carbon source: Usually glucose; provides energy and carbon skeletons.
Nitrogen source: Ammonium salts or nitrates for protein and nucleic acid synthesis.
Growth factors: Vitamins and minerals may be added to promote growth and metabolic activity.
These factors are continuously monitored in industrial fermenters using sensors connected to automated feedback systems to maintain optimum growth conditions.
Batch and Continuous Fermentation
Two primary methods are used in industrial-scale microbial cultivation: batch fermentation and continuous fermentation.
Batch Fermentation
In batch fermentation, microorganisms are added to a fixed amount of nutrient medium. No additional nutrients are supplied during the process. After growth ceases, the culture is harvested, and the bioreactor is cleaned before the next batch begins.
Advantages:
Simple and easy to control.
Suitable for production of secondary metabolites (e.g. antibiotics).
Reduced risk of contamination between batches.
Disadvantages:
Periods of downtime for cleaning and sterilisation.
Lower overall productivity due to non-continuous operation.
Continuous Fermentation
In continuous fermentation, fresh medium is continuously added to the bioreactor while culture liquid containing microorganisms and product is simultaneously removed.
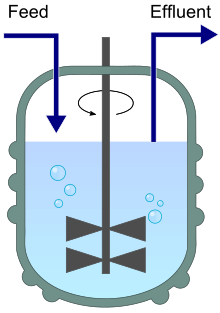
Chemostat schematic showing continuous feed and effluent that keep volume constant and sustain exponential growth. This configuration allows fine control of growth rate via dilution rate and supports high, steady productivity. Extra detail: impeller and aeration bubbles are shown to indicate mixing and oxygenation. Source.
Continuous Fermentation: A process where nutrients are constantly supplied, and product is continuously removed, maintaining microorganisms in the exponential phase of growth.
Advantages:
Maintains microbes in the log phase, where metabolic activity and productivity are highest.
Enables constant product output and high efficiency.
Useful for production of primary metabolites such as ethanol and enzymes.
Disadvantages:
Higher risk of contamination due to constant inflow and outflow.
More complex monitoring and control systems required.
Optimising Yield in Fermentation
To maximise product yield, several parameters are manipulated:
Temperature control ensures enzymes operate efficiently.
pH regulation maintains enzyme stability.
Oxygenation through spargers or paddles in aerobic processes.
Nutrient feed rate tailored to avoid limiting growth or causing inhibition.
Foam control using antifoam agents to prevent overflow and improve gas exchange.
The balance between growth and product formation depends on whether the target compound is a primary metabolite (produced during growth) or a secondary metabolite (produced after growth slows).
Monitoring and Yield Measurement
Efficient culturing relies on accurate monitoring:
Turbidity measurements using spectrophotometers estimate cell density.
Substrate and product concentrations are analysed to determine conversion efficiency.
Automated control systems adjust environmental variables in real time for optimal performance.
EQUATION
—-----------------------------------------------------------------
Specific Growth Rate (μ) = (ln N₂ – ln N₁) / (t₂ – t₁)
N₁ = Initial population size (cells cm⁻³)
N₂ = Final population size (cells cm⁻³)
t₁, t₂ = Times at the start and end of the growth interval (hours)
—-----------------------------------------------------------------
Understanding microbial growth kinetics allows biotechnologists to fine-tune conditions for maximum yield, ensuring efficient and cost-effective industrial production.
FAQ
The decision depends on the organism’s metabolic requirements and the desired product.
Aerobic conditions are used when oxygen is needed for respiration and biomass production, such as in enzyme synthesis or single-cell protein production.
Anaerobic conditions are used when oxygen inhibits product formation, such as in ethanol or lactic acid production.
Industrial fermenters include systems to monitor and adjust oxygen availability through spargers and agitators for precise control.
Foam traps microorganisms and reduces gas exchange, limiting oxygen supply and nutrient flow. It can also cause contamination if it escapes through exhaust vents.
Foam is controlled by:
Adding antifoam agents, such as silicone-based liquids or vegetable oils.
Mechanical foam breakers, which disrupt bubbles physically.
Regular monitoring ensures that foam control does not interfere with oxygen transfer or product purity.
Sensors continuously monitor parameters such as pH, temperature, oxygen, and nutrient concentration.
Data from these sensors feed into automated feedback systems, which adjust conditions in real time — for example:
Increasing aeration if oxygen drops.
Adding acid or alkali to stabilise pH.
Regulating temperature to maintain enzyme efficiency.
This automation keeps microorganisms in their optimal growth phase, maximising yield and reducing variability between production runs.
Stainless steel is non-reactive, durable, and easy to sterilise under high pressure and temperature.
Key benefits include:
Resistance to corrosion from acids or metabolic by-products.
Smooth interior surfaces, preventing microbial adhesion and biofilm formation.
Efficient heat transfer, allowing precise temperature control.
Glass and plastic are suitable only for small-scale laboratory cultures because they cannot withstand industrial sterilisation or continuous use.
Secondary metabolites, such as antibiotics, are typically produced when growth slows due to nutrient depletion.
As nutrients like nitrogen or carbon become scarce, microorganisms redirect metabolism from growth to stress-response pathways, leading to secondary metabolite synthesis.
This occurs during the stationary phase, when energy is used for defence, competition, or survival rather than cell division — a key consideration in optimising batch culture timing for maximum yield.
Practice Questions
Question 1 (2 marks)
Explain why aseptic technique is important when culturing microorganisms in biotechnology.
Mark scheme:
1 mark for stating that aseptic technique prevents contamination of cultures by unwanted microorganisms.
1 mark for stating that it ensures the validity of results or the purity and safety of the desired product.
Question 2 (5 marks)
Compare and contrast batch and continuous fermentation in the industrial production of microorganisms. In your answer, refer to the advantages and disadvantages of each method and how they affect product yield.
Mark scheme:
1 mark for stating that batch fermentation is a closed system where nutrients are not added after inoculation.
1 mark for stating that continuous fermentation involves continuous addition of nutrients and removal of culture.
1 mark for noting that batch fermentation is used for secondary metabolites (e.g. antibiotics) produced in the stationary phase, while continuous fermentation is suited for primary metabolites (e.g. ethanol, enzymes) produced during log phase.
1 mark for describing at least one advantage of batch fermentation (e.g. simple operation, reduced contamination risk).
1 mark for describing at least one advantage of continuous fermentation (e.g. high productivity, constant output, microbes maintained in exponential growth).

