OCR Specification focus:
‘Explain specificity, enzyme–substrate complexes, product formation and lowering of activation energy.’
Enzymes are biological catalysts that accelerate chemical reactions in living organisms by lowering activation energy. Their precise active sites ensure specificity, allowing only complementary substrates to bind and form enzyme–substrate complexes.
The Nature of Enzymes
Enzymes are globular proteins with unique three-dimensional structures vital for their catalytic function. Their shape arises from interactions between amino acid side chains, forming complex tertiary and quaternary structures that determine enzyme activity and specificity.
The Active Site
The active site is a small, specifically shaped region of an enzyme that directly interacts with the substrate. It is formed by the precise folding of the polypeptide chain, producing a unique arrangement of amino acid residues.
Active Site: The region of an enzyme with a specific shape that binds to the substrate and catalyses its conversion into product(s).
Only substrates with a complementary shape and chemical properties can bind to a given enzyme’s active site, explaining enzyme specificity — each enzyme typically acts on a single substrate or closely related group of substrates.
Enzyme–Substrate Complex Formation
When the substrate enters the active site, an enzyme–substrate complex forms. This complex stabilises the transition state and reduces the activation energy required for the reaction to proceed.
Enzyme–Substrate Complex: A temporary association formed when a substrate binds to an enzyme’s active site during catalysis.
Formation of this complex is central to the enzyme’s mechanism. Once the reaction occurs, product molecules are released, and the enzyme remains unchanged, ready to catalyse further reactions.
Enzyme Specificity
The specificity of an enzyme is determined by the shape, polarity, and charge distribution of its active site. This ensures that only substrates with complementary features bind successfully.
Specificity is maintained by non-covalent interactions such as:
Hydrogen bonds
Ionic interactions
Hydrophobic interactions
Van der Waals forces
If the enzyme’s shape is altered — for instance, by temperature or pH changes — the active site may no longer complement the substrate, leading to denaturation and loss of catalytic activity.
The Lock-and-Key Hypothesis
The lock-and-key model, proposed by Emil Fischer in 1894, was the first explanation of enzyme action and specificity. It suggests that the enzyme’s active site is a rigid structure perfectly complementary to its substrate.
Mechanism of Action
The enzyme (lock) possesses a fixed, rigid active site.
The substrate (key) fits precisely into this active site due to complementary shape and bonding interactions.
The enzyme–substrate complex forms, facilitating the conversion of substrate into product.
The product(s) no longer fit the active site and are released, leaving the enzyme free to bind another substrate.
This model effectively explains enzyme specificity but cannot fully describe observed flexibility in enzymes during catalysis.
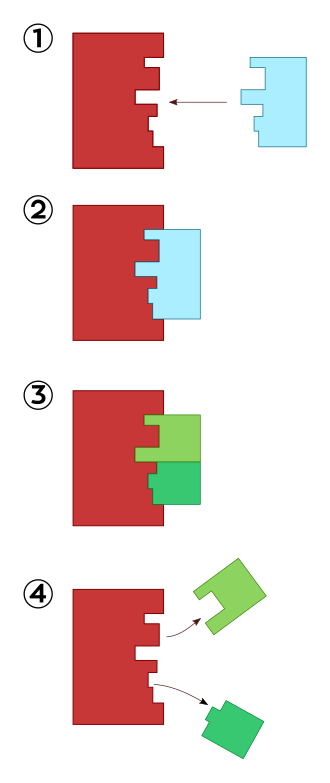
Lock-and-key model of enzyme action. The active site is pre-formed and rigid, accepting only a complementary substrate to form an enzyme–substrate complex before products are released. This diagram focuses on specificity and does not depict conformational change. Source.
The Induced Fit Hypothesis
To address the limitations of the lock-and-key model, Daniel Koshland proposed the induced fit hypothesis in 1958. This model recognises that enzymes are dynamic molecules, capable of subtle conformational changes upon substrate binding.
Mechanism of Induced Fit
The active site is not a perfect fit initially.
When the substrate binds, the enzyme changes shape slightly, moulding around the substrate.
This enhances the fit, stabilising the transition state and maximising interactions that lower activation energy.
After the reaction, the enzyme returns to its original conformation.
This flexibility ensures that enzymes are not rigid structures but adaptable catalysts capable of optimising binding and reaction conditions.
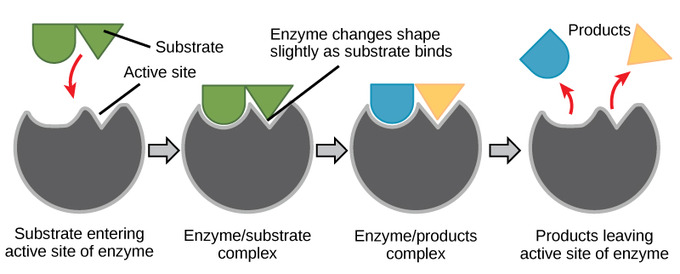
Induced-fit model: substrate binding reshapes the active site, producing a tighter, catalytic fit that stabilises the transition state and lowers activation energy. The enzyme relaxes to its original conformation after product release. If minor extra labels appear, they reinforce OCR-required ideas on catalysis. Source.
Lowering of Activation Energy
Every chemical reaction requires a minimum amount of energy, called the activation energy, to begin. Enzymes reduce this energy barrier by stabilising the transition state and weakening existing bonds in the substrate.
Activation Energy: The minimum amount of energy required for reactants to reach the transition state and form products in a chemical reaction.
Enzymes achieve this by:
Bringing substrates close together, increasing the likelihood of collision.
Orienting substrates correctly, aligning reactive groups optimally.
Providing an environment (e.g., acidic or hydrophobic) that facilitates bond breaking or formation.
Straining substrate bonds, making them easier to break.
EQUATION
—-----------------------------------------------------------------
Reaction Rate (v) = k [E][S]
v = rate of reaction (mol dm⁻³ s⁻¹)
k = rate constant dependent on temperature and enzyme
[E] = enzyme concentration (mol dm⁻³)
[S] = substrate concentration (mol dm⁻³)
—-----------------------------------------------------------------
By lowering activation energy, enzymes accelerate metabolic reactions that would otherwise occur too slowly to sustain life.
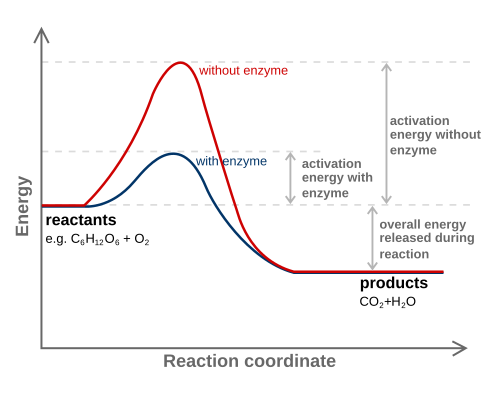
Reaction-coordinate diagram showing the higher activation energy of the uncatalysed pathway and the reduced barrier with a catalyst. Although generic, this is the standard visual used to illustrate how enzymes lower activation energy. Extra thermodynamic details beyond the OCR syllabus are minimal and can be ignored. Source.
Transition State Stabilisation
During catalysis, the substrate is converted to a transition state, an unstable intermediate form between substrate and product. Enzymes stabilise this transition state, reducing the energy required to reach it and facilitating its conversion into product.
Key stabilisation methods include:
Electrostatic interactions between charged residues and the transition state.
Proton transfer from active site residues to facilitate bond breaking.
Covalent catalysis, where a transient covalent bond forms between enzyme and substrate.
These mechanisms ensure reactions proceed rapidly and efficiently under physiological conditions.
Product Formation and Release
Once the reaction occurs, the enzyme forms an enzyme–product complex. Products typically have a lower affinity for the active site than the substrate, allowing them to dissociate easily.
Enzyme–Product Complex: The temporary association between an enzyme and its product molecules before their release from the active site.
The enzyme then reverts to its original state, ready for another catalytic cycle. This reusability is a defining feature of enzymes and underpins their efficiency in metabolism.
Summary of Mechanistic Concepts
Active site: region of catalysis with substrate specificity.
Lock-and-key model: enzyme has a fixed active site complementary to the substrate.
Induced fit model: enzyme’s active site changes shape for optimal binding.
Activation energy reduction: achieved through substrate orientation, bond strain, and transition state stabilisation.
Enzyme–substrate complex: key intermediate enabling rapid, specific catalysis.
FAQ
Temperature and pH alter the shape and charge distribution of the enzyme’s active site. Small changes can weaken hydrogen bonds and ionic interactions that maintain the enzyme’s tertiary structure.
If these interactions are disrupted, the active site may no longer be complementary to the substrate, reducing the rate of enzyme–substrate complex formation. Extreme conditions cause denaturation, permanently changing the active site and halting catalysis.
The degree of flexibility depends on the enzyme’s amino acid composition and tertiary structure. Enzymes with more loop regions and mobile domains can undergo larger conformational changes.
For example, enzymes involved in metabolic pathways often need flexibility to accommodate structurally similar substrates or multiple reaction steps, whereas highly specific enzymes such as DNA polymerase maintain tighter structural control to ensure precision.
Inhibitors may mimic substrates (competitive inhibitors) and initially induce a partial fit within the active site. However, because they lack the correct structure to reach the transition state, no catalysis occurs.
Non-competitive inhibitors bind to an allosteric site, altering the enzyme’s shape so that even if the substrate binds, the induced fit no longer forms correctly. This reduces or prevents transition state stabilisation, slowing or stopping the reaction.
The transition state represents a high-energy, short-lived intermediate where old bonds are partially broken and new bonds are forming.
Enzymes stabilise this state through:
Electrostatic interactions with charged residues in the active site
Straining substrate bonds to make them easier to break
Positioning functional groups optimally for reaction
Without this stabilisation, the activation energy would remain too high for the reaction to proceed at biological temperatures.
Yes. These models represent two ends of a continuum of enzyme behaviour rather than mutually exclusive mechanisms.
Some enzymes have largely pre-formed active sites fitting the lock-and-key model, but still undergo minor adjustments upon substrate binding, aligning more with the induced fit model.
This hybrid view reflects modern structural biology findings showing that enzyme dynamics are fine-tuned rather than fixed, combining both precision and flexibility for efficient catalysis.
Practice Questions
Question 1 (2 marks)
Explain why enzymes are described as having specificity.
Mark Scheme:
1 mark for stating that enzyme specificity arises because the active site has a complementary shape to its substrate.
1 mark for explaining that only one type of substrate (or a group of closely related substrates) can bind to the enzyme’s active site to form an enzyme–substrate complex.
Question 2 (5 marks)
Describe and compare the lock-and-key and induced fit models of enzyme action, and explain how each model accounts for enzyme specificity and catalysis.
Mark Scheme:
1 mark for stating that the lock-and-key model suggests the enzyme’s active site is rigid and complementary in shape to the substrate before binding.
1 mark for describing that the substrate fits exactly into the active site, forming an enzyme–substrate complex (ES complex).
1 mark for stating that the induced fit model suggests the active site changes shape slightly to fit around the substrate upon binding.
1 mark for explaining that this conformational change stabilises the transition state and helps lower activation energy.
1 mark for comparison: both models explain specificity of enzymes, but the induced fit model better accounts for enzyme flexibility and catalytic efficiency.

