OCR Specification focus:
‘Compare competitive, non-competitive, reversible and irreversible inhibition; explain end-product inhibition.’
Enzyme Inhibition and Regulation
Enzymes are biological catalysts that control the rate of metabolic reactions within cells. To maintain metabolic balance, enzyme activity must be precisely regulated. Regulation often occurs through enzyme inhibition, where molecules reduce or prevent enzyme activity. Understanding inhibition and regulation is essential for explaining how cells coordinate biochemical pathways.
Types of Enzyme Inhibition
Competitive Inhibition
Competitive inhibition occurs when an inhibitor molecule has a similar shape to the substrate and competes for the enzyme’s active site. Because it binds to the same site, it prevents substrate molecules from forming enzyme–substrate complexes.
The effect is reversible because the inhibitor can be displaced if substrate concentration increases.
Competitive Inhibitor: A molecule structurally similar to the substrate that binds temporarily to an enzyme’s active site, preventing substrate binding.
Key characteristics:
The inhibitor and substrate compete for the same active site.
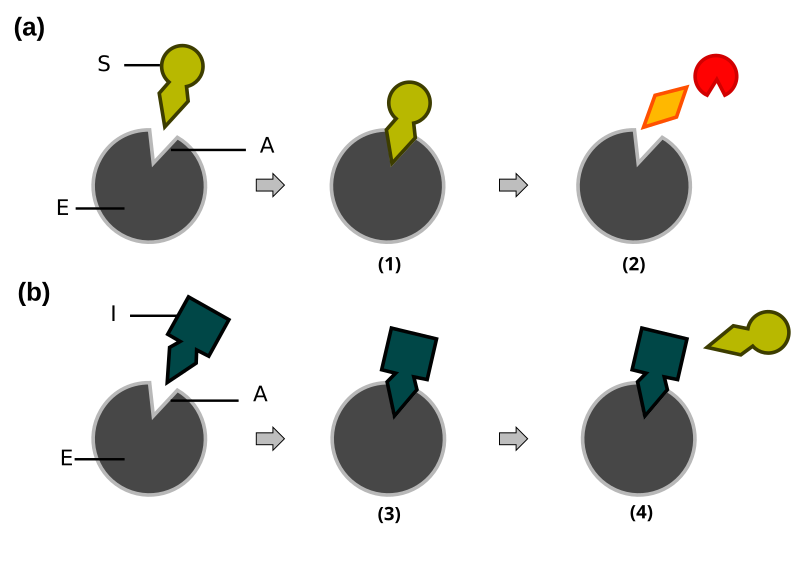
A labelled diagram of competitive inhibition illustrating that an inhibitor with a similar shape to the substrate binds the active site, preventing enzyme–substrate complex formation. Increasing substrate concentration can outcompete the inhibitor, so Vmax is unchanged while apparent Km increases. The visual reflects the reversible, active-site mechanism described in the notes. Source.
Increasing substrate concentration reduces the degree of inhibition.
The maximum rate of reaction (Vmax) is unchanged, but apparent affinity (Km) increases because more substrate is needed to reach half Vmax.
Example: Malonate competes with succinate for the active site of succinate dehydrogenase in respiration.
This reversible competition provides a flexible means of controlling enzyme-catalysed reactions in metabolic pathways.
Non-Competitive Inhibition
In non-competitive inhibition, the inhibitor binds to an allosteric site—a region other than the active site—causing a conformational change in the enzyme. This alters the shape of the active site so the substrate can no longer bind effectively.
Allosteric Site: A specific region on an enzyme distinct from the active site where a molecule binds, causing a change in enzyme shape and function.
Key characteristics:
The inhibitor does not compete with the substrate for the active site.
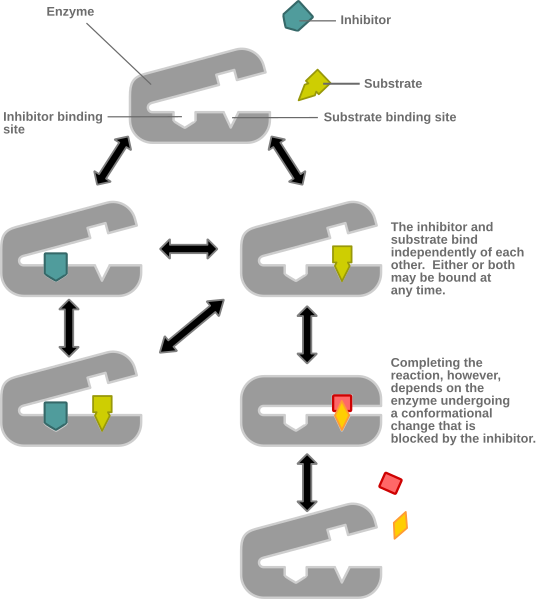
A schematic of non-competitive inhibition in which an inhibitor binds an allosteric site, changing the active site’s shape so substrate no longer binds effectively. Because binding is away from the active site, increasing substrate concentration does not overcome inhibition, and Vmax decreases while Km is unchanged. Labels clearly show enzyme, substrate, inhibitor, and altered conformation. Source.
Increasing substrate concentration does not reduce inhibition.
The enzyme’s Vmax decreases, but the Km remains unchanged.
Example: Cyanide inhibits the enzyme cytochrome oxidase in the electron transport chain by altering the active site shape.
Non-competitive inhibition allows fine control of metabolic activity because enzyme function can be modulated without directly blocking the active site.
Reversible and Irreversible Inhibition
Enzyme inhibition can be classified further by whether the inhibitor’s effects can be undone.
Reversible Inhibition
Reversible inhibitors bind non-covalently (e.g. hydrogen or ionic bonds) and can dissociate from the enzyme, restoring activity when removed. Both competitive and non-competitive inhibitors can be reversible.
Reversible inhibition allows temporary regulation of enzyme activity.
The degree of inhibition depends on relative concentrations of inhibitor and substrate.
Irreversible Inhibition
Irreversible inhibitors form covalent bonds with the enzyme, causing permanent loss of activity.
The inhibitor usually modifies amino acid residues in the active site.
The enzyme must be re-synthesised for activity to return.
Example: Aspirin irreversibly inhibits cyclooxygenase (COX), blocking prostaglandin synthesis and reducing inflammation.
Example: Organophosphates, used in pesticides, permanently inhibit acetylcholinesterase, leading to nerve malfunction.
Irreversible Inhibitor: A molecule forming a permanent covalent bond with an enzyme, rendering it catalytically inactive.
Irreversible inhibitors are often potent toxins or pharmaceutical agents designed for targeted enzyme suppression.
Enzyme Regulation in Metabolic Pathways
Cells coordinate thousands of biochemical reactions simultaneously. Enzyme regulation ensures that metabolic pathways function efficiently, preventing accumulation or depletion of intermediates. Regulation often occurs through feedback mechanisms that sense and adjust enzyme activity in response to cellular needs.
End-Product Inhibition
End-product inhibition is a key example of negative feedback control in metabolism. The final product of a multi-step pathway acts as an inhibitor of an enzyme that catalyses an earlier step.
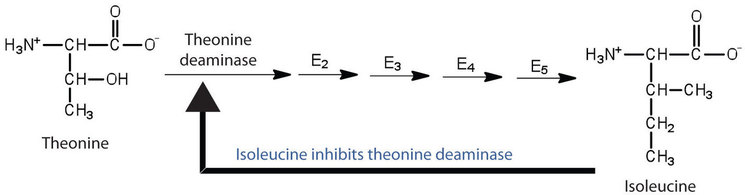
Pathway diagram for isoleucine biosynthesis showing the end product isoleucine binding allosterically to threonine deaminase to down-regulate flux — a textbook case of negative feedback via non-competitive inhibition. The figure is clearly labelled with pathway steps and the inhibitory arrow from isoleucine to the first enzyme. Note: the intermediate steps are shown for context; these extra labels exceed the minimal OCR detail but help students anchor the mechanism to a real pathway. Source.
This prevents overproduction of the end product and conserves cellular resources.
End-Product Inhibition: Regulation in which the final product of a metabolic pathway inhibits an enzyme involved earlier in the pathway, reducing its own synthesis rate.
Key features:
The product binds reversibly to an enzyme’s allosteric site.
As product concentration increases, inhibition strengthens, slowing the pathway.
When product concentration falls, inhibition is released, and enzyme activity resumes.
This provides automatic self-regulation of metabolic flux.
Example:
In isoleucine biosynthesis from threonine, the end product isoleucine inhibits threonine deaminase, the first enzyme in the pathway. When isoleucine levels are high, it binds allosterically, preventing excessive production.
End-product inhibition ensures metabolic homeostasis, maintaining constant internal conditions and preventing unnecessary energy expenditure.
Allosteric Regulation and Conformational Changes
Many enzymes possess multiple allosteric sites that enable fine-tuned regulation. Allosteric activators stabilise the enzyme’s active conformation, while inhibitors stabilise an inactive form.
Allosteric activators: Increase enzyme affinity for substrate and speed up catalysis.
Allosteric inhibitors: Decrease enzyme activity by altering the active site.
Cooperativity in multi-subunit enzymes (e.g. haemoglobin) allows activity to respond sharply to small changes in substrate concentration.
This flexible system enables rapid adjustment to metabolic demand without altering gene expression or enzyme synthesis rates.
Physiological Importance of Enzyme Inhibition
Enzyme inhibition plays essential roles in metabolic control, drug action, and toxin effects:
Metabolic regulation: Maintains homeostasis through feedback loops.
Pharmaceutical targeting: Many drugs act as enzyme inhibitors (e.g. statins inhibit HMG-CoA reductase to lower cholesterol).
Defence mechanisms: Natural toxins (e.g. snake venom phospholipases) act by irreversibly inhibiting vital enzymes.
In biological systems, inhibition and regulation represent sophisticated strategies for maintaining efficiency and stability across complex biochemical networks.
FAQ
Reversible inhibitors form non-covalent bonds such as hydrogen, ionic, or hydrophobic interactions, allowing them to bind and release easily. Their effects are temporary and depend on the concentration of substrate and inhibitor.
Irreversible inhibitors, in contrast, form covalent bonds with amino acid residues in the enzyme, permanently altering the active site. This means the enzyme’s structure and function are lost until it is re-synthesised by the cell.
End-product inhibition involves the final product binding to an allosteric site rather than the active site. This binding triggers a conformational change that alters the enzyme’s active site and decreases its affinity for the substrate.
This mechanism allows feedback control of metabolic pathways. It ensures that when sufficient product accumulates, the enzyme’s activity slows down automatically, maintaining efficient resource use and metabolic balance.
If end-product inhibition failed, enzymes in the pathway would continue catalysing reactions unchecked. This would lead to:
Overproduction of the final product.
Depletion of substrates and intermediates needed in other pathways.
Loss of homeostasis, potentially causing metabolic imbalances.
In some cases, accumulation of the product may also become toxic or waste cellular energy, emphasising the importance of this regulatory mechanism.
Enzyme inhibitors are used therapeutically to regulate metabolic reactions linked to disease. Examples include:
Statins: Competitive inhibitors of HMG-CoA reductase, reducing cholesterol synthesis.
ACE inhibitors: Block angiotensin-converting enzyme, lowering blood pressure.
Protease inhibitors: Used in antiviral therapy to prevent viral protein maturation.
By mimicking natural substrates or binding to allosteric sites, inhibitors can precisely target malfunctioning metabolic pathways in humans.
The inhibitor’s shape and binding affinity determine its mode of inhibition.
If its shape closely resembles the substrate, it fits the active site, acting as a competitive inhibitor.
If it binds elsewhere on the enzyme, changing the active site’s shape, it acts as a non-competitive inhibitor.
Additionally, some molecules can switch between mechanisms depending on enzyme conformation or environmental conditions, showing how dynamic enzyme regulation can be.
Practice Questions
Question 1 (2 marks)
Explain how a competitive inhibitor affects the rate of an enzyme-catalysed reaction and how increasing substrate concentration influences this effect.
Mark scheme:
1 mark: States that a competitive inhibitor has a similar shape to the substrate and binds to the enzyme’s active site, preventing substrate binding.
1 mark: Explains that increasing substrate concentration reduces the inhibitor’s effect because the substrate outcompetes the inhibitor for the active site.
Question 2 (5 marks)
Describe and compare the mechanisms of non-competitive inhibition and end-product inhibition in enzyme-controlled reactions.
Mark scheme:
1 mark: States that in non-competitive inhibition, the inhibitor binds to an allosteric site, not the active site.
1 mark: Explains that this causes a conformational change in the enzyme, altering the active site so the substrate can no longer bind effectively.
1 mark: Notes that increasing substrate concentration does not overcome non-competitive inhibition.
1 mark: Describes that end-product inhibition is a type of non-competitive inhibition where the final product of a metabolic pathway inhibits an earlier enzyme.
1 mark: Explains that this provides negative feedback control, preventing overproduction of the end product and conserving resources.

