OCR Specification focus:
‘Investigate temperature and pH effects on rate; include the temperature coefficient, Q10.’
Enzymes are biological catalysts whose activity depends heavily on environmental conditions. Temperature and pH are critical factors influencing enzyme structure, stability, and reaction rate, determining optimal catalytic efficiency.
Temperature and Enzyme Activity
Kinetic Influence of Temperature
Enzymes accelerate reactions by lowering activation energy. Increasing temperature raises the kinetic energy of molecules, enhancing the frequency of successful enzyme–substrate collisions. This typically increases the rate of reaction up to an optimum temperature. Beyond this point, enzyme stability decreases rapidly.
At low temperatures, molecular motion is reduced; fewer collisions occur, resulting in low reaction rates.
As temperature rises, kinetic energy increases, so more enzyme–substrate complexes form per unit time.
Above the optimum temperature (commonly around 37°C for human enzymes), the rate drops sharply due to denaturation.
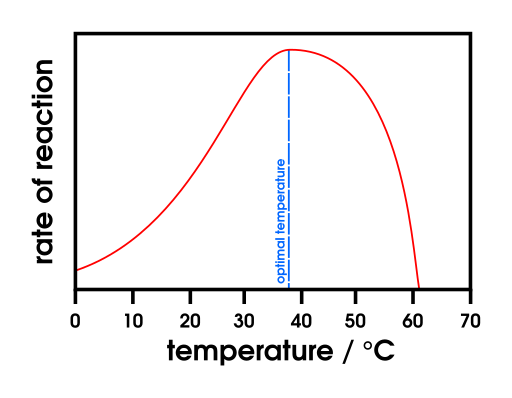
Graph showing a typical enzyme activity–temperature relationship: increasing rate with temperature to an optimum, followed by sharp decline as denaturation disrupts the active site. The bell-shaped profile reflects the balance between kinetic gain and thermal instability. This schematic matches general enzyme behaviour rather than a specific dataset. Source.
Denaturation and Loss of Enzyme Function
Denaturation involves the disruption of an enzyme’s tertiary structure, altering the shape of the active site so substrates can no longer bind effectively.
Denaturation: The irreversible alteration of an enzyme’s three-dimensional structure due to the breaking of hydrogen bonds and other interactions, leading to loss of catalytic activity.
Heat breaks hydrogen bonds, ionic bonds, and hydrophobic interactions maintaining the enzyme’s conformation. Because the primary structure (amino acid sequence) remains intact, renaturation is rare but possible if conditions return to normal before full denaturation.
Temperature Coefficient (Q10)
EQUATION
—-----------------------------------------------------------------
Temperature Coefficient (Q10) = Rate of reaction at (T + 10°C) ÷ Rate of reaction at T°C
Q10 = Ratio showing how much the rate of reaction increases with a 10°C temperature rise.
—-----------------------------------------------------------------
For most enzyme-catalysed reactions, Q10 ≈ 2 up to the optimum temperature, meaning the rate roughly doubles for every 10°C rise. Beyond the optimum, Q10 falls because denaturation overtakes kinetic benefit.
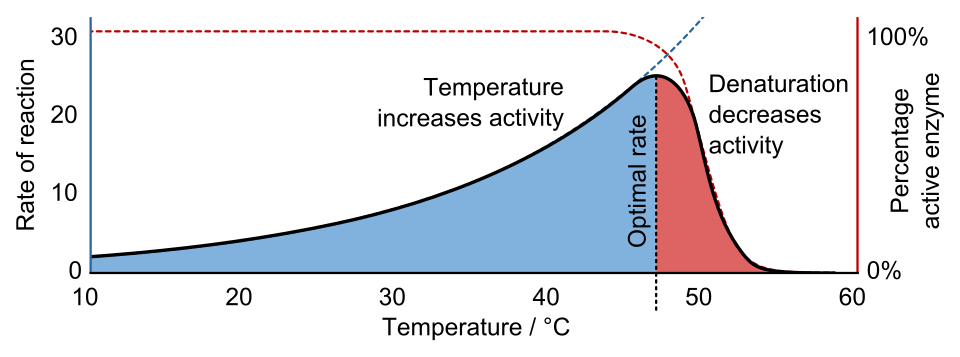
Conceptual illustration of Q10: rate increases with temperature (left), the fraction of folded enzyme drops above a threshold (middle), and overall activity peaks at an intermediate optimum (right). The “fraction folded” panel is an extra detail not required by OCR but clarifies why activity ultimately falls. Source.
Enzyme Stability at Extremes of Temperature
Some enzymes are adapted to extreme conditions:
Thermophilic enzymes (e.g. from Thermus aquaticus) remain stable at 70°C due to additional ionic bonds and hydrophobic interactions.
Psychrophilic enzymes (cold-adapted organisms) have more flexible structures, allowing function at low temperatures but reduced stability at higher ones.
Such adaptations demonstrate the importance of molecular flexibility and bonding patterns in determining enzyme thermostability.
pH and Enzyme Activity
Role of pH in Enzyme Function
The pH of a solution reflects its hydrogen ion concentration [H⁺]. Enzymes contain ionisable side chains, particularly on amino acids such as aspartic acid and lysine, whose charge depends on environmental pH. These charged residues influence both the enzyme’s tertiary structure and the active site’s ability to interact with substrates.
pH: A logarithmic measure of hydrogen ion concentration, defined as pH = −log₁₀[H⁺]. Low pH indicates high [H⁺] (acidic), while high pH indicates low [H⁺] (alkaline).
Every enzyme has an optimum pH, at which the active site maintains the correct charge and shape for binding.
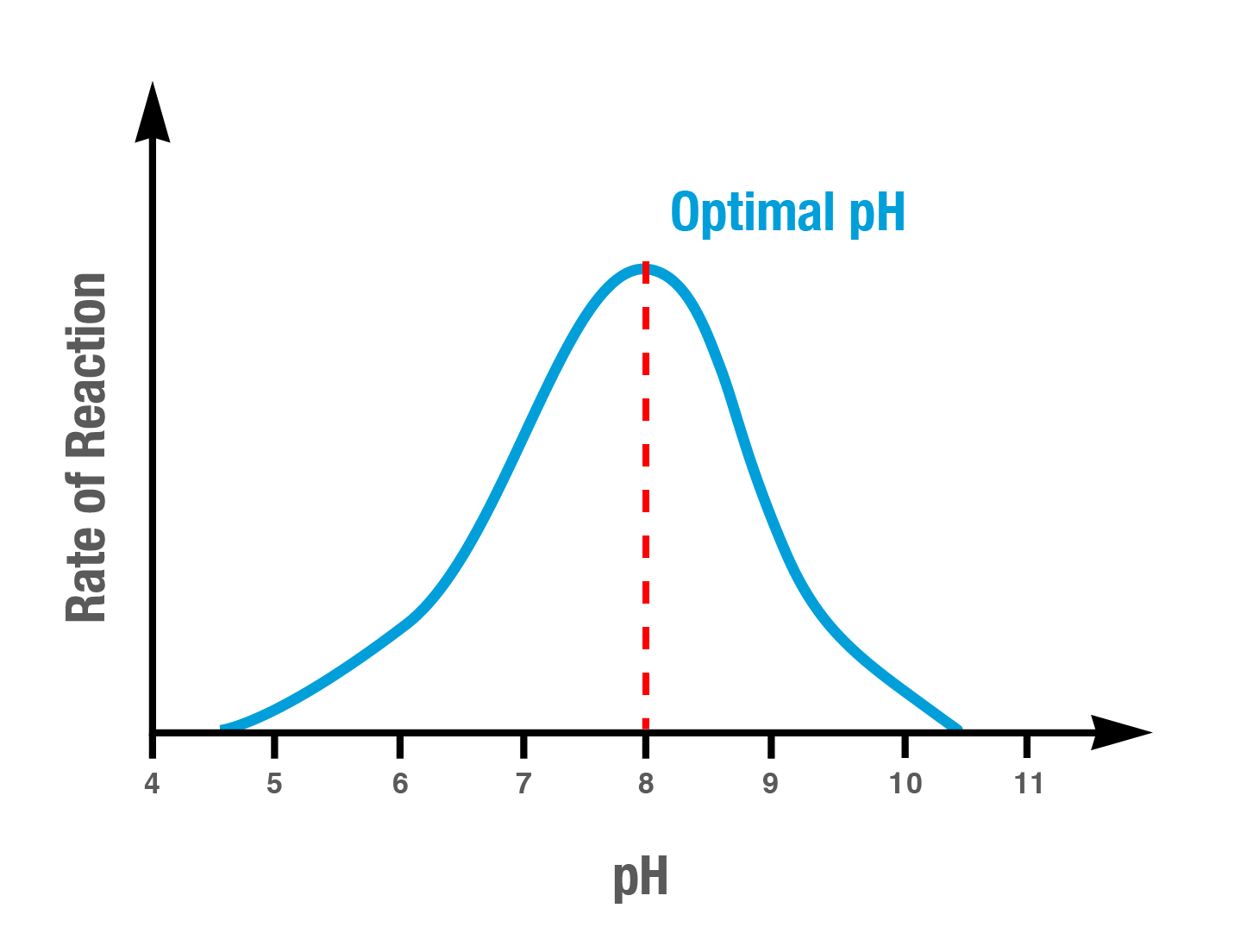
Graph of enzyme activity vs pH, peaking at an optimum pH and falling at more acidic or alkaline values as ionisation changes disrupt active-site charge and tertiary structure. This schematic aligns with typical enzyme profiles; exact values vary by enzyme. Source.
Low or high pH disrupts ionic and hydrogen bonds maintaining tertiary structure.
This alters the active site’s geometry, reducing substrate affinity.
Extreme deviations cause irreversible denaturation.
Examples of pH Optima
Pepsin (a stomach protease) operates optimally at pH 2, suited to the acidic gastric environment.
Amylase, secreted into the mouth, functions best at pH 7.
Trypsin, an intestinal enzyme, has an optimum around pH 8.
These examples reflect how enzymes are adapted to the pH of their natural environments, ensuring efficient catalysis.
Molecular Basis of pH Effects
Changes in pH influence the ionisation state of amino acid R groups:
Altered R group charge may prevent substrate binding if ionic attraction is disrupted.
Shifts in charge can also distort the active site’s tertiary configuration, hindering enzyme–substrate complex formation.
When the environmental pH returns to normal, minor conformational changes may be reversible, but severe pH stress causes permanent denaturation.
Buffer Systems in Enzyme Experiments
In laboratory investigations, buffer solutions are used to maintain constant pH, ensuring observed rate changes are due solely to other variables (e.g. temperature). Accurate control of pH allows meaningful interpretation of enzyme kinetics.
Investigating Temperature and pH Effects
Practical Approach
When investigating temperature and pH:
Keep enzyme and substrate concentrations constant to ensure a fair test.
Measure the rate of product formation (e.g. oxygen from catalase) or substrate depletion.
Use colorimetric or gas volume methods to quantify reaction rates.
Conduct trials across a range of controlled temperatures or pH values.
Data Interpretation
Graphical analysis typically produces bell-shaped curves:
The ascending part reflects increased kinetic energy or favourable ionisation.
The descending part indicates denaturation or charge disruption.
For temperature studies, students should calculate Q10 values for intervals within the linear region to assess proportional rate changes.
Importance of Temperature and pH Regulation in Organisms
Enzymes underpin all metabolic reactions; thus, maintaining optimal temperature and pH is vital for life. In homeostasis, physiological mechanisms stabilise these factors:
Thermoregulation ensures enzyme stability and reaction efficiency in fluctuating environments.
Buffer systems in blood (e.g. carbonic acid–hydrogencarbonate) stabilise pH to prevent enzyme malfunction.
Disturbances such as fever, hypothermia, or acidosis lead to reduced enzyme activity, slowing essential metabolic processes. Understanding these dependencies underpins physiological and biochemical knowledge essential to OCR A-Level Biology.
FAQ
The Q10 coefficient quantifies how temperature affects enzyme reaction rates, allowing comparison of temperature sensitivity between species.
A higher Q10 indicates greater temperature dependence — typical for enzymes from cold environments.
A lower Q10 suggests stability across a wider temperature range, often seen in thermophilic organisms.
By comparing Q10 values, biologists can infer evolutionary adaptations to environmental temperature.
Excess thermal energy disrupts hydrogen bonds, ionic bonds, and hydrophobic interactions that maintain the enzyme’s tertiary structure.
As these bonds break, the protein unfolds, and the active site loses its complementary shape. This prevents substrate binding, halting catalysis.
Denaturation is usually irreversible because the complex folding pattern cannot be restored once disrupted.
The decrease in activity is typically steeper on one side of the optimum because the rate of charge alteration on amino acid residues is not uniform.
Near the acidic side, excessive hydrogen ions may protonate negatively charged groups rapidly.
On the alkaline side, deprotonation may occur more gradually.
This asymmetry reflects the differing rates of structural disruption as pH deviates from the enzyme’s optimum environment.
Buffers contain a weak acid and its conjugate base (or vice versa). When small amounts of acid or alkali are added, the buffer system reacts to neutralise changes in hydrogen ion concentration.
Added acid (H⁺) is absorbed by the conjugate base.
Added alkali (OH⁻) is neutralised by the weak acid.
This balance maintains a nearly constant pH, ensuring that observed changes in enzyme activity result from temperature or substrate variation, not fluctuating pH.
Optimum temperature depends on the stability and bonding within each enzyme’s structure. Enzymes adapted to different environments evolve variations in the number and type of bonds stabilising their tertiary structures.
Thermophilic enzymes from heat-loving organisms contain extra ionic and disulphide bonds that resist denaturation at high temperatures.
Psychrophilic enzymes have fewer stabilising interactions, allowing flexibility at low temperatures but causing instability at moderate heat.
These structural adaptations determine how temperature influences enzyme activity and stability.
Practice Questions
Question 1 (2 marks)
Describe how increasing temperature affects the rate of an enzyme-controlled reaction up to and beyond the enzyme’s optimum temperature.
Mark Scheme:
(1 mark) Increasing temperature raises kinetic energy, leading to more frequent and successful enzyme–substrate collisions up to the optimum temperature.
(1 mark) Beyond the optimum, enzymes denature as hydrogen bonds and ionic interactions break, altering the active site and reducing reaction rate.
Question 2 (5 marks)
Explain how pH affects the activity of enzymes and describe how buffers can be used to control pH in enzyme investigations.
Mark Scheme:
(1 mark) pH affects the ionisation of amino acid side chains in the enzyme and substrate.
(1 mark) Changes in pH alter charges on the active site, affecting enzyme–substrate complex formation.
(1 mark) Extreme pH values disrupt hydrogen and ionic bonds, causing denaturation and loss of tertiary structure.
(1 mark) Each enzyme has an optimum pH where its active site is the correct shape for binding.
(1 mark) Buffers maintain a stable pH by resisting changes in hydrogen ion concentration, allowing enzyme activity to be measured accurately.

