OCR Specification focus:
‘Explain the need for cofactors and coenzymes; include chloride ions and vitamins as examples.’
Cofactors and coenzymes are essential for the optimal functioning of enzymes, acting as non-protein helpers that enable catalysis, structural stability, and metabolic flexibility across biological systems.
The Role of Cofactors in Enzyme Function
Enzymes are biological catalysts composed mainly of proteins. However, many enzymes require additional non-protein components to function effectively. These non-protein substances are known as cofactors, and they can be either inorganic ions or organic molecules. Without their specific cofactors, certain enzymes may be completely inactive or exhibit greatly reduced catalytic activity.
Definition of Cofactor
Cofactor: A non-protein chemical substance that must be bound to an enzyme for the enzyme to function efficiently.
Cofactors can assist in various ways, including:
Stabilising the enzyme’s structure, ensuring correct folding or charge distribution.
Participating directly in chemical reactions, such as electron transfer or substrate binding.
Acting as temporary carriers for atoms or groups removed or added during reactions.
Cofactors are crucial in metabolic processes, particularly in respiration, photosynthesis, and synthesis of macromolecules.
Types of Cofactors
Cofactors are divided into three main categories:
Inorganic ions (inorganic cofactors)
Coenzymes (organic cofactors)
Prosthetic groups (a subset of tightly bound coenzymes)
Inorganic Cofactors
Inorganic cofactors are metal ions or non-carbon-based ions that temporarily bind to enzymes or substrates to improve catalytic efficiency.
Examples include:
Chloride ions (Cl⁻) – required for the activity of the enzyme amylase, which breaks down starch into maltose. The chloride ions act by inducing a conformational change in the enzyme’s active site, optimising substrate binding.
Zinc ions (Zn²⁺) – essential for the enzyme carbonic anhydrase, which catalyses the reversible reaction between carbon dioxide and water to form carbonic acid.
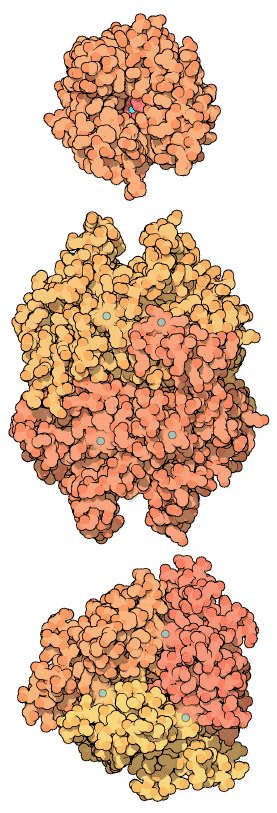
Carbonic anhydrase active sites: representative α, β and γ carbonic anhydrases with Zn²⁺ at the catalytic centre. The metal ion coordinates key residues and water, lowering activation energy for CO₂ hydration. Extra structural variety across classes is shown but is not required learning for this subsubtopic. Source.
Magnesium ions (Mg²⁺) – vital for stabilising structures of ATP and nucleotides, and for the activity of enzymes involved in phosphorylation reactions.
These inorganic cofactors do not undergo permanent chemical change during the reaction; they assist the enzyme transiently.
Coenzymes: Organic Cofactors Derived from Vitamins
Definition of Coenzyme
Coenzyme: A small, organic, non-protein molecule that binds temporarily to an enzyme and participates in the reaction, often transferring chemical groups or electrons.
Coenzymes often function as intermediary carriers, shuttling atoms or functional groups between enzymes in metabolic pathways. They are not consumed permanently and can be reused repeatedly after regeneration.
Most coenzymes are derived from vitamins, particularly the B-group vitamins, which act as precursors.
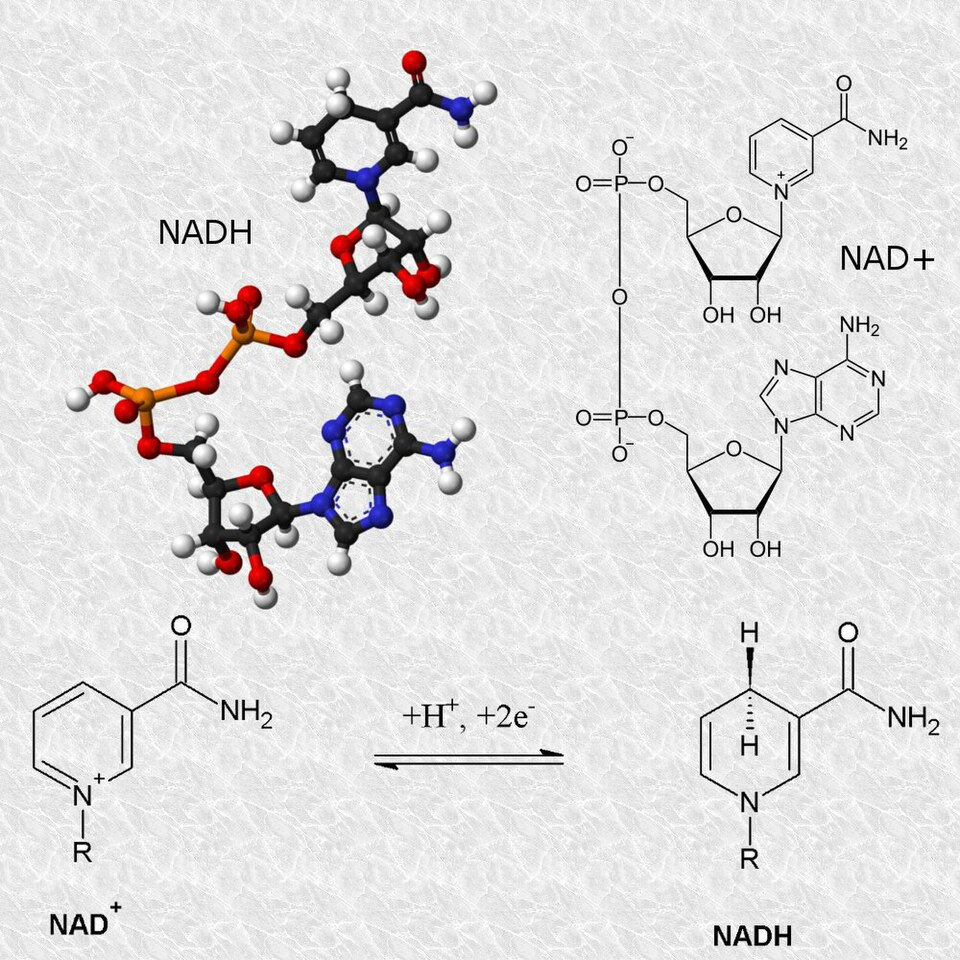
NAD/NADH redox pair: the coenzyme cycles between oxidised NAD⁺ and reduced NADH while carrying electrons and hydrogen during metabolic pathways such as respiration. This highlights the regenerative, carrier role of coenzymes. The image focuses on form, not detailed reaction mechanisms, which are beyond this subsubtopic. Source.
A deficiency in a vitamin can therefore impair enzyme function and disrupt metabolism.
Examples of coenzymes and their vitamin sources:
NAD (nicotinamide adenine dinucleotide) – derived from vitamin B₃ (niacin); functions in redox reactions during respiration, alternating between oxidised (NAD⁺) and reduced (NADH) forms.
FAD (flavin adenine dinucleotide) – derived from vitamin B₂ (riboflavin); functions similarly in redox processes within the Krebs cycle.
Coenzyme A (CoA) – derived from vitamin B₅ (pantothenic acid); essential in the transfer of acetyl groups to form acetyl coenzyme A, a critical intermediate in carbohydrate and lipid metabolism.
After each catalytic cycle, coenzymes are regenerated to their original form, maintaining continuous metabolic flux.
Prosthetic Groups: Permanently Bound Cofactors
Definition of Prosthetic Group
Prosthetic group: A tightly bound, permanent non-protein component of an enzyme that is essential to its structure and catalytic function.
Unlike coenzymes, prosthetic groups remain permanently attached to the enzyme, often via covalent bonding. They play a structural and functional role, enabling redox reactions and stabilising transition states.
Example:
Haem group in cytochrome oxidase and catalase enzymes.
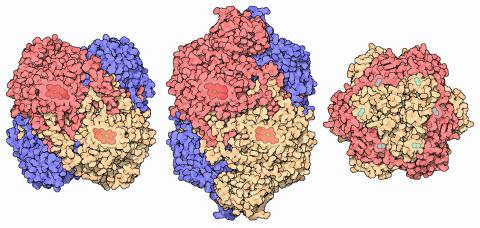
Catalase with haem/iron prosthetic group: human catalase is a tetramer whose haem (Fe) groups enable rapid decomposition of hydrogen peroxide. The gallery also contrasts bacterial variants, including a manganese-containing catalase; these comparative details exceed OCR requirements but reinforce the concept of prosthetic groups. Source.
Prosthetic groups often define the enzyme’s specificity and reactivity, forming part of the active site’s essential chemistry.
Mechanisms of Cofactor Action
Cofactors influence enzyme activity through several mechanisms:
Altering enzyme shape – enabling the active site to adopt a catalytically competent conformation.
Forming part of the active site – directly participating in substrate binding or catalysis.
Acting as electron or group carriers – transferring chemical species between substrates in multistep reactions.
Stabilising charged intermediates – reducing activation energy and increasing reaction rate.
These mechanisms ensure high specificity and efficiency, critical in complex biological systems.
Relationship Between Cofactors, Coenzymes and Vitamins
Many vitamins act as precursors for coenzymes and are therefore essential micronutrients. A lack of vitamins leads to deficiencies that impair enzyme-catalysed pathways:
Vitamin B₁ (thiamine) deficiency leads to accumulation of pyruvate due to reduced activity of pyruvate dehydrogenase.
Vitamin B₁₂ deficiency affects the synthesis of nucleotides and red blood cells.
In this way, vitamins are biochemical building blocks for coenzymes, linking nutrition directly to enzyme function.
Enzyme–Cofactor Relationships
Certain enzymes exist as inactive apoenzymes (enzyme without cofactor). Upon binding to the required cofactor, they become active holoenzymes.
Apoenzyme: The inactive protein component of an enzyme that requires a cofactor to function.
Holoenzyme: The active enzyme formed when an apoenzyme binds to its cofactor.
This dynamic interplay allows cellular control over enzyme activity, preventing unnecessary reactions when cofactors are scarce or withheld.
Biological Significance of Cofactors and Coenzymes
Cofactors are fundamental for metabolic integration and regulation. They:
Enable electron transport in respiration and photosynthesis.
Facilitate energy transfer through ATP synthesis.
Support biosynthetic and degradative pathways.
Allow fine-tuning of enzyme specificity and control.
Without cofactors and coenzymes, enzyme systems could not sustain life’s intricate biochemical networks, illustrating their indispensable role in biology.
FAQ
The nature of a cofactor depends on the type of reaction the enzyme catalyses.
Metal ions such as Zn²⁺, Mg²⁺, and Fe²⁺ stabilise negative charges or assist in electron transfer, particularly in oxidation–reduction reactions.
Organic molecules (coenzymes), often derived from vitamins, act as carriers of specific chemical groups (e.g. hydrogen, acetyl) between enzymes.
Metal ions mainly stabilise structures or charges, while coenzymes act as mobile group carriers in metabolic pathways.
Cofactors can subtly alter an enzyme’s active site shape or charge distribution, improving binding to its specific substrate.
In some enzymes, a cofactor helps form the correct three-dimensional conformation of the active site. In others, it stabilises the transition state of the reaction, allowing only a particular substrate to bind efficiently.
Thus, cofactors can refine enzyme specificity by ensuring that only the correct substrate interacts productively with the catalytic site.
Yes, certain enzymes depend on multiple cofactors for full functionality.
For instance, pyruvate dehydrogenase uses several coenzymes and ions, including NAD, FAD, Coenzyme A, and Mg²⁺. Each performs a specific role, such as transferring electrons, acetyl groups, or stabilising charged intermediates.
Having multiple cofactors allows the enzyme complex to catalyse multi-step reactions efficiently within a single catalytic sequence.
Coenzymes often act in reversible redox or transfer reactions, becoming modified temporarily.
They are regenerated when they participate in subsequent reactions:
NADH is reoxidised to NAD⁺ during oxidative phosphorylation.
Coenzyme A is released after transferring its acetyl group to another molecule.
This continuous regeneration allows a small pool of coenzymes to support many cycles of enzymatic reactions without being depleted.
Both remain associated with enzymes, but prosthetic groups are permanently integrated, often via covalent bonds, while tightly bound coenzymes can detach under certain conditions.
For example, the haem group in catalase is a true prosthetic group, structurally embedded within the enzyme. In contrast, biotin in carboxylase enzymes, though strongly bound, can dissociate and reattach.
This distinction affects how enzymes are classified and how they respond to environmental or structural changes.
Practice Questions
Question 1 (2 marks)
Explain the difference between a cofactor and a coenzyme, giving one example of each.
Mark Scheme:
1 mark for stating that a cofactor is a non-protein substance required by an enzyme for activity.
1 mark for correctly distinguishing that a coenzyme is an organic cofactor, often derived from a vitamin, and giving suitable examples (e.g. chloride ion for a cofactor; NAD or FAD for a coenzyme).
(Maximum 2 marks)
Question 2 (5 marks)
Describe and explain the roles of cofactors and coenzymes in enzyme-controlled reactions. Include at least one named example in your answer.
Mark Scheme:
1 mark for stating that cofactors are non-protein helpers that enable enzymes to function.
1 mark for explaining that inorganic cofactors, such as metal ions, temporarily bind to enzymes or substrates to assist catalysis (e.g. chloride ions for amylase, zinc ions for carbonic anhydrase).
1 mark for describing coenzymes as organic cofactors that act as carriers of chemical groups or electrons during reactions (e.g. NAD transferring hydrogen during respiration).
1 mark for recognising that coenzymes are derived from vitamins, and deficiencies can reduce enzyme activity.
1 mark for explaining that some cofactors, such as prosthetic groups (e.g. haem in catalase), are permanently bound and form part of the enzyme’s active site.
(Maximum 5 marks)

