OCR Specification focus:
‘Outline cholinergic synapse structure; neurotransmitter action; the roles of summation; and inhibitory versus excitatory synapses.’
Synapses are specialised junctions that enable communication between neurones or between a neurone and an effector cell, ensuring rapid, coordinated responses essential for nervous system function.
Structure of a Synapse
A synapse is the point where one neurone communicates with another or with an effector. The small gap between the two cells is called the synaptic cleft, typically around 20–40 nm wide.
Main Components
Presynaptic neurone – transmits the impulse towards the synapse; its end forms the presynaptic knob, rich in mitochondria and synaptic vesicles containing neurotransmitters.
Synaptic cleft – the extracellular gap separating the two membranes, across which neurotransmitters diffuse.
Postsynaptic neurone (or cell) – has a postsynaptic membrane containing specific receptor proteins complementary to the neurotransmitter.
Neurotransmitter: A chemical messenger released from a neurone that diffuses across the synaptic cleft to stimulate or inhibit another cell.
Adaptations of the Presynaptic Knob
Contains numerous mitochondria to provide ATP for neurotransmitter synthesis and vesicle recycling.
Has voltage-gated calcium ion channels that open in response to an arriving action potential.
Possesses synaptic vesicles that store neurotransmitters such as acetylcholine in cholinergic synapses.
Contains enzymes and transport proteins to synthesise and recycle neurotransmitters efficiently.
Transmission Across a Cholinergic Synapse
A cholinergic synapse uses the neurotransmitter acetylcholine (ACh). It is the most common type in the mammalian nervous system, especially at neuromuscular junctions.
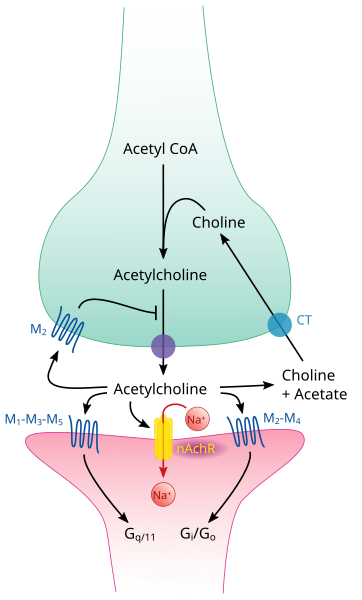
Labeled diagram of a cholinergic synapse showing Ca²⁺-triggered vesicle fusion, ACh release into the synaptic cleft, binding to postsynaptic ligand-gated ion channels, and AChE hydrolysis with choline re-uptake. Demonstrates one-way transmission and neurotransmitter clearance. Extra detail includes presynaptic M₂ autoreceptors beyond OCR’s required depth. Source.
Sequence of Events
Arrival of an action potential
The impulse reaches the presynaptic knob, causing voltage-gated calcium channels to open.
Calcium ion influx
Ca²⁺ ions diffuse into the presynaptic knob down their electrochemical gradient.
Vesicle fusion and neurotransmitter release
Calcium ions stimulate synaptic vesicles to fuse with the presynaptic membrane.
Acetylcholine is released into the synaptic cleft by exocytosis.
Diffusion and receptor binding
ACh diffuses across the cleft and binds to specific receptor sites on the postsynaptic membrane, usually associated with ligand-gated sodium ion channels.
Postsynaptic depolarisation
Binding causes these sodium channels to open, allowing Na⁺ ions to enter, generating an excitatory postsynaptic potential (EPSP).
If the EPSP reaches the threshold potential, a new action potential is generated in the postsynaptic neurone.
Neurotransmitter breakdown
The enzyme acetylcholinesterase (AChE), located in the synaptic cleft, rapidly hydrolyses ACh into choline and ethanoic acid (acetate).
This prevents continuous stimulation of the postsynaptic membrane.
Recycling of neurotransmitter components
Choline and acetate are reabsorbed into the presynaptic neurone, recombined to form ACh using ATP, and stored in vesicles for reuse.
Excitatory postsynaptic potential (EPSP): A small, temporary depolarisation of the postsynaptic membrane caused by Na⁺ ion influx following neurotransmitter binding.
This highly controlled process ensures one-way transmission, as only the presynaptic neurone has vesicles and the postsynaptic membrane possesses receptor sites.
Summation
When individual EPSPs are subthreshold, they can combine to trigger an action potential. This process is known as summation.
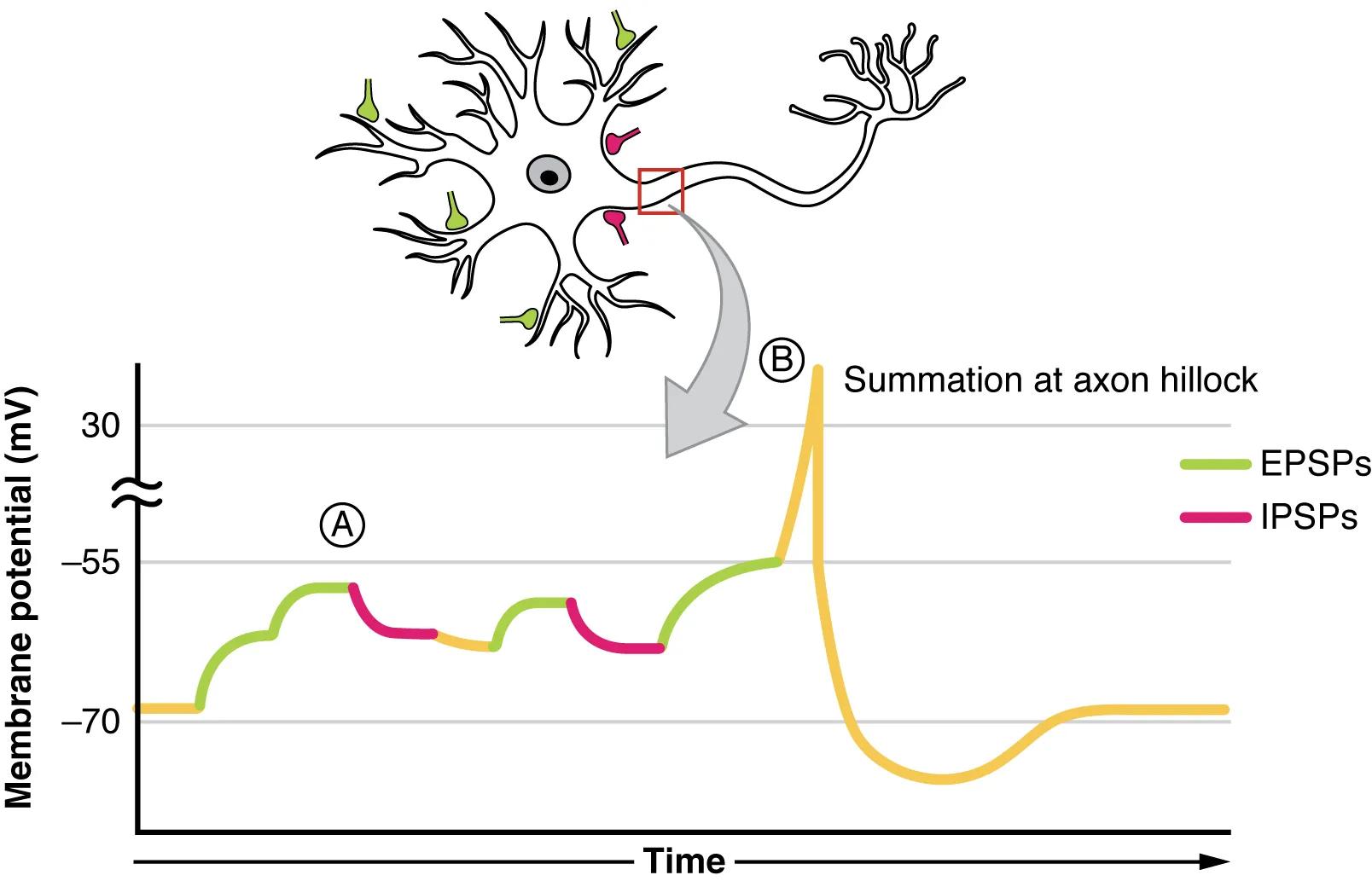
Graphical illustration of postsynaptic potential summation showing how multiple EPSPs can depolarise the membrane to threshold and how IPSPs counteract excitation. Highlights spatial and temporal summation mechanisms relevant to OCR-level understanding. Source.
Types of Summation
Temporal summation – Several impulses arrive in quick succession from the same presynaptic neurone, allowing their combined EPSPs to reach threshold.
Spatial summation – Simultaneous impulses from multiple presynaptic neurones combine their EPSPs on a single postsynaptic neurone.
These mechanisms enhance the flexibility and sensitivity of neural circuits, allowing integration of signals from various sources.
Inhibitory Synapses
Not all synapses are excitatory. Inhibitory synapses reduce the likelihood of an action potential being generated.
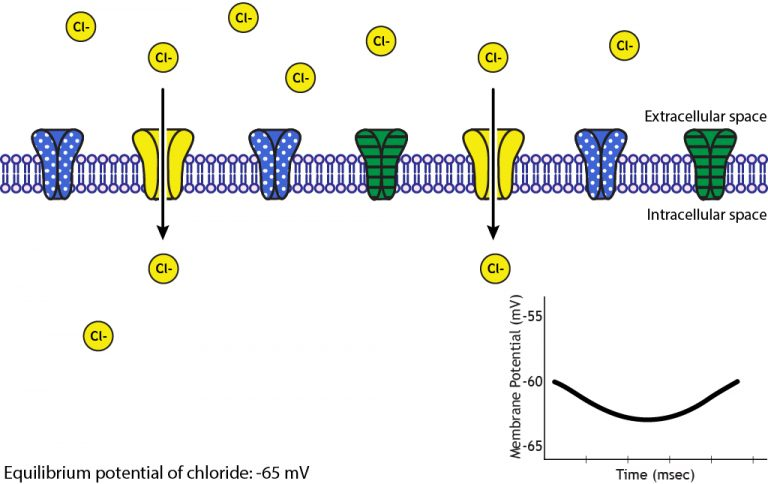
Illustration of IPSP generation showing opening of chloride channels that allow Cl⁻ influx, hyperpolarising the membrane and reducing the likelihood of an action potential. Depicts ligand-gated Cl⁻ channel action; extra page details on reversal potentials exceed OCR scope. Source.
Mechanism of Inhibition
The neurotransmitter (for example GABA, gamma-aminobutyric acid) binds to receptors on the postsynaptic membrane.
This opens chloride (Cl⁻) or potassium (K⁺) channels, causing either influx of Cl⁻ ions or efflux of K⁺ ions.
The membrane becomes hyperpolarised (more negative inside), making it less likely to reach threshold potential.
Inhibitory postsynaptic potential (IPSP): A temporary hyperpolarisation of the postsynaptic membrane that reduces the probability of an action potential.
The balance of EPSPs and IPSPs determines whether the postsynaptic neurone fires, allowing fine-tuned control of neural activity.
Comparison: Excitatory vs Inhibitory Synapses
Excitatory synapses: Depolarise the postsynaptic membrane; neurotransmitters open sodium ion channels; may trigger action potentials.
Inhibitory synapses: Hyperpolarise the postsynaptic membrane; neurotransmitters open chloride or potassium ion channels; prevent depolarisation.
The integration of both types provides neural pathway modulation, enabling complex control of behaviour and movement.
Importance of Synapses in the Nervous System
Synapses are vital for information processing and coordination within the nervous system. They perform several essential functions:
Ensure unidirectional transmission of impulses.
Allow divergence, where one neurone influences several others, amplifying responses.
Permit convergence, where multiple neurones combine inputs for a coordinated response.
Enable filtering of weak stimuli through summation thresholds.
Provide sites for synaptic plasticity, crucial for learning and memory.
The chemical and electrical events at synapses underpin every aspect of nervous system activity, from reflexes to conscious thought.
FAQ
The speed of synaptic transmission depends mainly on the rate of neurotransmitter release, diffusion, and breakdown.
A greater number of vesicles or faster exocytosis increases transmission rate.
The width of the synaptic cleft affects how quickly acetylcholine diffuses across.
The efficiency of acetylcholinesterase (AChE) in hydrolysing acetylcholine determines how rapidly the synapse resets.
Temperature, ion concentration, and the metabolic activity (ATP availability) of the presynaptic knob also influence transmission speed.
Some drugs and toxins mimic, block, or alter normal neurotransmitter action:
Agonists (e.g., nicotine) bind to acetylcholine receptors, stimulating the postsynaptic membrane.
Antagonists (e.g., curare) block receptors, preventing depolarisation.
AChE inhibitors (e.g., nerve gas, some pesticides) prevent breakdown of acetylcholine, causing continuous stimulation and muscle paralysis.
Calcium channel blockers reduce vesicle fusion and neurotransmitter release.
These agents demonstrate how delicate the synaptic balance is for proper nerve function.
Synaptic transmission is always one-way because of structural and chemical specialisation.
Only the presynaptic neurone contains vesicles filled with neurotransmitters.
Only the postsynaptic membrane has receptor proteins specific to those neurotransmitters.
This design ensures impulses flow from one neurone to the next in a coordinated and predictable manner, maintaining clear signal pathways within complex neural networks.
Inhibitory synapses are vital for controlling excitatory activity and preventing overstimulation.
They generate inhibitory postsynaptic potentials (IPSPs), hyperpolarising the postsynaptic membrane.
This balances excitatory input (EPSPs), shaping neuronal firing patterns.
In the brain, this selective inhibition allows precise control of motor responses, filtering of sensory input, and prevention of excessive neuronal firing that could lead to seizures or loss of coordination.
After neurotransmitter release, vesicle membranes are retrieved and reused through endocytosis.
Portions of the presynaptic membrane are pinched off to form new vesicles.
These vesicles are refilled with neurotransmitter, often using ATP-driven transport.
This recycling conserves materials and energy, maintaining synaptic efficiency during repetitive or high-frequency stimulation.
If vesicle recycling fails, neurotransmitter supply drops, impairing transmission and leading to synaptic fatigue.
Practice Questions
Question 1 (2 marks)
Explain how the enzyme acetylcholinesterase (AChE) contributes to the proper functioning of a cholinergic synapse.
Mark Scheme:
1 mark: States that AChE breaks down acetylcholine (ACh) into choline and ethanoic acid (acetate).
1 mark: Explains that this prevents continuous stimulation of the postsynaptic membrane, allowing the synapse to reset for the next impulse.
Question 2 (5 marks)
Describe and explain how information is transmitted across a cholinergic synapse, and how the postsynaptic neurone is prevented from being continuously stimulated.
Mark Scheme:
1 mark: Action potential arrives at the presynaptic knob, causing voltage-gated calcium ion channels to open.
1 mark: Calcium ions diffuse into the presynaptic knob and trigger synaptic vesicles to fuse with the presynaptic membrane.
1 mark: Acetylcholine (ACh) is released by exocytosis and diffuses across the synaptic cleft.
1 mark: ACh binds to specific receptor sites on the postsynaptic membrane, opening sodium ion channels and causing depolarisation (EPSP).
1 mark: Acetylcholinesterase breaks down ACh, stopping stimulation; choline is reabsorbed and recycled to form new ACh.

