OCR Specification focus:
‘Explain control of gene expression at transcription: the lac operon model in bacteria and the general role of transcription factors in eukaryotes.’
Gene expression is tightly regulated at the transcriptional level, ensuring proteins are synthesised only when required. Both prokaryotes and eukaryotes employ specialised mechanisms to achieve efficient control.
Transcriptional Control Overview
Transcriptional control determines whether a gene is transcribed into messenger RNA (mRNA). By regulating transcription initiation, cells conserve energy and resources, producing proteins only when necessary.
Importance of Transcriptional Regulation
Ensures cell specialisation and differentiation in multicellular organisms.
Allows cells to respond to environmental changes rapidly.
Prevents unnecessary synthesis of enzymes or structural proteins.
Transcriptional control: The regulation of gene expression by controlling the initiation and rate of transcription of DNA into mRNA.
Gene regulation occurs differently in prokaryotes (bacteria) and eukaryotes, reflecting their contrasting cellular organisation and complexity.
The Lac Operon in Bacteria
Structure of the Lac Operon
The lac operon is a classic example of transcriptional control in Escherichia coli, a bacterium that uses glucose preferentially but can metabolise lactose when glucose is absent.
It consists of:
Structural genes: lacZ, lacY, and lacA, encoding enzymes for lactose metabolism.
Promoter (P): A DNA sequence where RNA polymerase binds to initiate transcription.
Operator (O): A regulatory DNA sequence that binds the repressor protein.
Regulatory gene (lacI): Codes for the lac repressor, a protein that inhibits transcription.
Operon: A cluster of genes under the control of a single promoter and operator, transcribed together as a single mRNA molecule.
The lac operon illustrates how bacteria adjust gene expression based on nutrient availability, maximising metabolic efficiency.
Function of the Lac Operon
The operon operates under two main conditions:
1. Lactose absent (glucose present)
The lac repressor binds to the operator, blocking RNA polymerase from transcribing structural genes.
Enzymes for lactose metabolism are not produced, conserving resources.
2. Lactose present (glucose absent)
Lactose (or its isomer allolactose) binds to the repressor, altering its shape so it cannot bind the operator.
RNA polymerase binds to the promoter and transcribes lacZ, lacY, and lacA.
The resulting enzymes, including β-galactosidase, break down lactose into glucose and galactose.
This process demonstrates inducible control, where gene expression is switched on by the presence of a substrate.
Inducible enzyme: An enzyme whose synthesis is initiated in response to the presence of a specific substrate.
Role of cAMP and the CAP Complex
Even when lactose is present, maximum transcription of the lac operon occurs only when glucose levels are low. This additional layer of control ensures glucose remains the preferred energy source.
When glucose levels fall, the concentration of cyclic AMP (cAMP) increases.
cAMP binds to catabolite activator protein (CAP), forming a complex.
The cAMP–CAP complex binds to the promoter region, increasing RNA polymerase’s affinity for the promoter.
Transcription of the lac genes is greatly enhanced.
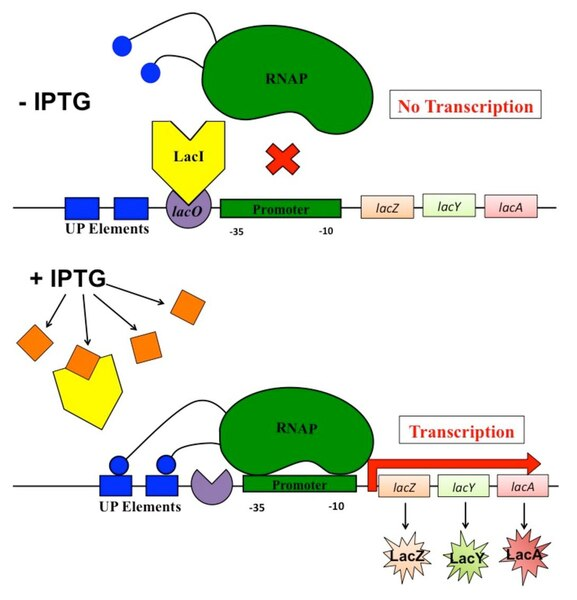
Transcriptional regulation of the lac operon in E. coli. The diagram shows lacI repressor control at the operator and CAP–cAMP activation near the promoter, coordinating induction by lactose and catabolite repression by glucose. Minor extras such as precise binding site labels are included but remain within OCR scope. Source.
cAMP (cyclic adenosine monophosphate): A secondary messenger molecule that activates CAP, promoting transcription when glucose is scarce.
This mechanism exemplifies dual control—negative control via the repressor and positive control via the CAP–cAMP complex—allowing E. coli to fine-tune gene expression.
Transcriptional Control in Eukaryotes
Chromatin Structure and Accessibility
In eukaryotes, transcription occurs in the nucleus, and gene accessibility is influenced by chromatin organisation.
Heterochromatin: Densely packed DNA; transcriptionally inactive.
Euchromatin: Loosely packed DNA; accessible to RNA polymerase.
Modification of histone proteins by acetylation or methylation alters DNA–protein interactions and influences whether a gene is expressed.
Histone acetylation: The addition of acetyl groups to histone proteins, reducing DNA binding and promoting transcriptional activation.
Histone acetylation typically enhances transcription, whereas deacetylation and DNA methylation repress gene activity.
Transcription Factors
Transcription factors (TFs) are crucial proteins that regulate gene expression by controlling the binding of RNA polymerase II to promoter regions.
There are two main types:
Activator transcription factors: Increase the rate of transcription by helping RNA polymerase bind.
Repressor transcription factors: Decrease transcription by blocking RNA polymerase binding or by recruiting repressive complexes.
Transcription factor: A protein that binds to specific DNA sequences to control the rate of transcription of genetic information.
Each transcription factor recognises a specific promoter or enhancer sequence, enabling precise spatial and temporal gene expression.
Mechanism of Transcription Factor Function
Signal molecules (such as hormones or growth factors) activate transcription factors through phosphorylation or conformational change.
Activated transcription factors enter the nucleus and bind to regulatory DNA sequences upstream of target genes.
This binding either recruits RNA polymerase II or inhibits its attachment, depending on the factor type.
The process integrates multiple intracellular and extracellular signals, ensuring that genes are expressed at the right time and in the correct tissues.
For example, steroid hormones like oestrogen bind to intracellular receptors that act as transcription factors, stimulating gene expression relevant to cell growth and metabolism.
Combinatorial Control
Most eukaryotic genes are regulated by multiple transcription factors acting together. This combinatorial approach allows for:
Fine-tuned gene expression.
Tissue-specific expression patterns.
Integration of various environmental and developmental cues.
Such complexity provides flexibility and precision, ensuring that organisms can adapt and develop properly.
Enhancers and Silencers
Enhancers: DNA sequences that, when bound by activator proteins, increase transcription levels even when located far from the promoter.
Silencers: DNA elements that bind repressor proteins, reducing transcription efficiency.
The three-dimensional folding of DNA brings these regions close to promoters, allowing coordinated regulation over long genomic distances.
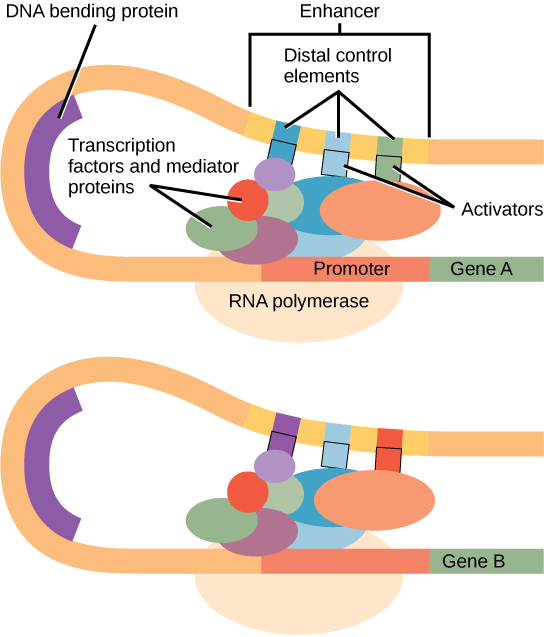
Eukaryotic transcriptional activation by enhancers: activators bound at distal control elements interact with mediator and RNA polymerase II at the promoter through DNA looping. This captures how multiple transcription factors integrate signals to regulate initiation. The figure includes mediator and distal elements—appropriate detail for OCR students. Source.
Summary of Key Concepts
The lac operon exemplifies prokaryotic transcriptional control using repressors and activators.
Transcription factors and chromatin modification underpin eukaryotic control mechanisms.
Both systems demonstrate how organisms regulate gene expression efficiently in response to internal and external stimuli.
FAQ
The lac operon in E. coli uses two regulatory mechanisms:
Negative control occurs when the lac repressor binds to the operator, preventing RNA polymerase from transcribing structural genes. This blocks transcription when lactose is absent.
Positive control happens when glucose is low, leading to an increase in cAMP. The cAMP binds to CAP, forming a complex that attaches to the promoter, enhancing RNA polymerase binding and increasing transcription.
Together, these mechanisms ensure that the operon is only fully active when lactose is present and glucose is absent.
An inducible operon is switched on by the presence of a substrate that inactivates a repressor protein.
In the lac operon, lactose (or allolactose) acts as the inducer. When lactose is available, it binds to the lac repressor, causing a conformational change that prevents the repressor from binding to the operator. This allows transcription to occur.
In contrast, a repressible operon (e.g. the trp operon) is normally active and switched off when its product binds to the repressor, stopping transcription.
Transcription factors are often regulated by cell signalling pathways. External signals, such as hormones or growth factors, trigger intracellular cascades that modify transcription factors.
Phosphorylation by protein kinases can activate or deactivate transcription factors.
Ligand binding, such as hormone-receptor interaction, can change a transcription factor’s shape or localisation.
Some transcription factors remain inactive in the cytoplasm until they receive a signal to move into the nucleus.
These mechanisms ensure that genes respond precisely to environmental or developmental cues.
Promoters are DNA sequences located immediately upstream of a gene. They serve as binding sites for RNA polymerase and general transcription factors, marking where transcription begins.
Enhancers are distant regulatory sequences that can be located thousands of base pairs away. They bind activator transcription factors, which interact with promoter-bound complexes through DNA looping.
While promoters are essential for transcription initiation, enhancers fine-tune expression levels, ensuring genes are transcribed in the right cells at the right time.
Chromatin remodelling determines whether genes are accessible to transcription machinery. DNA is wrapped around histone proteins, forming nucleosomes.
Histone acetylation reduces histone–DNA attraction, opening the chromatin and promoting transcription.
Histone deacetylation and DNA methylation tighten chromatin structure, silencing gene expression.
This reversible process allows cells to regulate which genes are expressed during development or in response to stimuli, providing a key mechanism for differential gene expression.
Practice Questions
Question 1 (3 marks)
Describe how the lac operon in E. coli is regulated when lactose is present and glucose is absent.
Mark Scheme:
1 mark: Lactose (or allolactose) binds to the repressor protein, causing it to change shape so it can no longer bind to the operator.
1 mark: RNA polymerase binds to the promoter and transcribes the structural genes (lacZ, lacY, lacA).
1 mark: The CAP–cAMP complex binds near the promoter, increasing RNA polymerase activity and enhancing transcription.
Question 2 (6 marks)
Compare the control of gene expression in the bacterial lac operon and in eukaryotic cells.
Mark Scheme:
1 mark: The lac operon is an example of transcriptional control in prokaryotes where genes are organised in an operon (cluster of genes under one promoter).
1 mark: It involves negative control by a repressor protein and positive control by the CAP–cAMP complex.
1 mark: Transcription occurs only when lactose is present and glucose is absent, showing environmental responsiveness.
1 mark: In eukaryotes, genes are controlled individually, not in operons.
1 mark: Transcription factors regulate transcription by binding to promoters, enhancers, or silencers to activate or repress gene expression.
1 mark: Eukaryotic control is influenced by chromatin structure (acetylation/methylation) and allows cell differentiation and tissue-specific expression.

