OCR Specification focus:
‘Describe how proteins can be activated after translation, including phosphorylation cascades triggered by cyclic AMP second messenger pathways.’
Post-Translational Control: Activation by cAMP
Cells finely regulate protein activity after translation to ensure precise control of metabolism, growth, and signalling. Cyclic AMP (cAMP) plays a key role in this activation process.
Overview of Post-Translational Control
After translation, many proteins are inactive precursors requiring modification to become functional. Post-translational control refers to the regulation of protein activity after the polypeptide chain has been synthesised by a ribosome. These modifications enable rapid cellular responses to internal and external stimuli.
Main Forms of Post-Translational Modification
Phosphorylation – addition of phosphate groups to amino acids, often activating or deactivating enzymes.
Methylation – addition of methyl groups affecting protein–DNA interactions.
Acetylation – modification of lysine residues influencing protein structure and function.
Glycosylation – addition of carbohydrate chains that alter stability or targeting.
Proteolytic cleavage – removal of peptide fragments to activate precursor proteins (e.g. insulin activation).
Among these, phosphorylation cascades triggered by cAMP are particularly important in coordinating enzyme activity.
The Role of Cyclic AMP (cAMP)
Cyclic adenosine monophosphate (cAMP) is a secondary messenger — a small intracellular molecule that transmits signals from extracellular stimuli such as hormones. It enables rapid amplification of a signal without the signalling molecule entering the cell.
Secondary messenger: A molecule that relays signals from receptors on the cell surface to target molecules inside the cell, triggering a physiological response.
Production of cAMP
Many hormones (e.g. adrenaline, glucagon) bind to G protein-coupled receptors (GPCRs) on the plasma membrane.
This activates a G protein, which in turn stimulates adenylate cyclase, an enzyme embedded in the membrane.
Adenylate cyclase converts ATP (adenosine triphosphate) into cAMP.
cAMP diffuses through the cytoplasm, initiating a phosphorylation cascade that regulates enzyme activity.
EQUATION
—-----------------------------------------------------------------
Formation of cAMP
ATP → cAMP + PPi
ATP = Adenosine triphosphate, high-energy nucleotide
cAMP = Cyclic adenosine monophosphate, secondary messenger
PPi = Inorganic pyrophosphate
—-----------------------------------------------------------------
This reaction is catalysed by adenylate cyclase and is reversed by phosphodiesterase, which breaks cAMP down to AMP, thus stopping the signal.
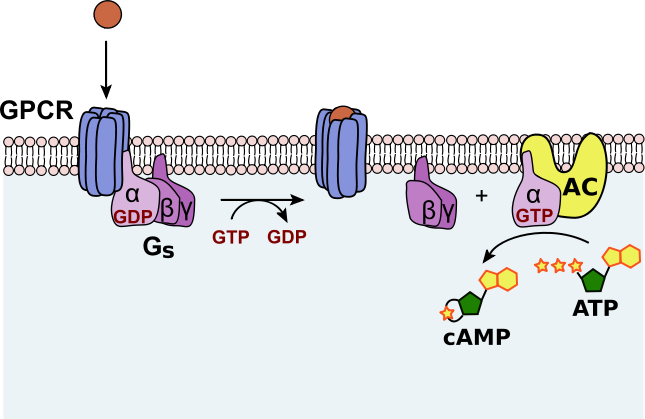
Activation of a G-protein-coupled receptor (GPCR) stimulates adenylate cyclase, converting ATP to cAMP. Rising cAMP levels initiate a phosphorylation cascade by activating downstream kinases such as PKA. The diagram is simplified to emphasise the GPCR–Gαs–adenylate cyclase–cAMP sequence appropriate for OCR A-Level. Source.
Phosphorylation Cascades Triggered by cAMP
Once generated, cAMP activates protein kinase A (PKA), also known as cAMP-dependent protein kinase.
Activation of Protein Kinase A (PKA)
In its inactive state, PKA consists of two regulatory and two catalytic subunits.
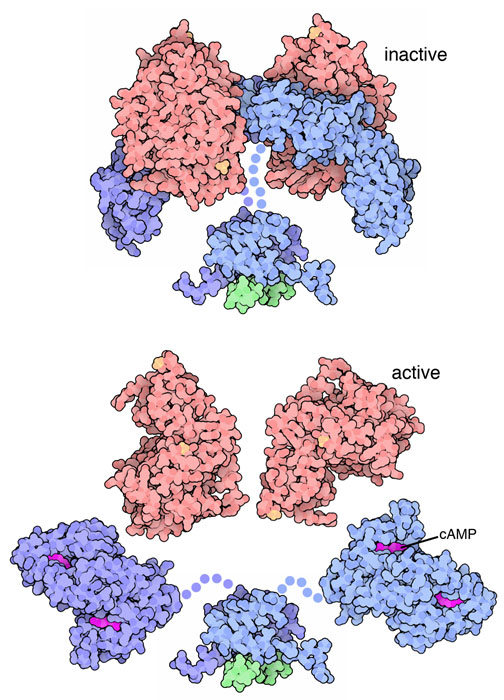
When cAMP binds the regulatory subunits, PKA releases its catalytic subunits, which then phosphorylate target proteins. The upper panel shows the inactive tetramer; the lower panel shows the active, dissociated state. The illustration includes a small AKAP peptide (green) that localises PKA—extra detail beyond the OCR requirement but helpful context for cellular targeting. Source.
cAMP binds to the regulatory subunits, causing them to change shape and release the catalytic subunits.
The free catalytic subunits are now active and phosphorylate specific target proteins.
These target proteins include enzymes involved in metabolic pathways, transcription factors, and other protein kinases, leading to a cascade effect where one activated molecule triggers many downstream reactions.
Key Features of Phosphorylation Cascades
Amplification: A single signalling molecule can lead to the activation of thousands of enzymes.
Reversibility: Phosphorylation is reversible through the action of phosphatases, ensuring tight control.
Specificity: Different cell types express unique sets of kinases and targets, creating precise outcomes.
Phosphorylation usually involves the transfer of a phosphate group from ATP to a serine, threonine, or tyrosine residue within the protein structure.
Phosphorylation: The covalent addition of a phosphate group to an amino acid residue of a protein, altering its shape and activity.
This modification changes the conformation of the protein, either activating or inhibiting its function depending on the site and context of phosphorylation.
Example: The cAMP Pathway in Glucose Metabolism
An important example of post-translational activation by cAMP occurs in liver and muscle cells during the “fight or flight” response initiated by adrenaline.
Step-by-Step Process
Adrenaline binds to a β-adrenergic receptor on the cell membrane.
The receptor activates a G protein, which then activates adenylate cyclase.
Adenylate cyclase converts ATP into cAMP.
cAMP binds to PKA, activating it.
PKA phosphorylates glycogen phosphorylase kinase, activating it.
Glycogen phosphorylase kinase phosphorylates glycogen phosphorylase, the enzyme that breaks down glycogen into glucose-1-phosphate.
Through this cascade, a small extracellular signal (adrenaline) leads to a large intracellular response — rapid release of glucose for respiration.
Control and Regulation of cAMP Pathways
Because of its potency, cAMP signalling must be tightly regulated to prevent excessive activation.
Key Regulatory Mechanisms
Phosphodiesterase (PDE) rapidly degrades cAMP to AMP, terminating the signal.
Feedback inhibition: Some downstream proteins inhibit earlier steps in the cascade.
Compartmentalisation: Enzymes and signalling molecules are localised in specific cell regions, preventing unwanted cross-talk between pathways.
Drugs such as caffeine inhibit phosphodiesterase, prolonging the effects of cAMP and increasing alertness through sustained enzyme activation.
Broader Biological Significance
The cAMP-mediated phosphorylation system is a universal signalling mechanism found across many organisms. It enables cells to:
Respond rapidly to changing environments without synthesising new proteins.
Integrate multiple signals to coordinate metabolism, growth, and gene expression.
Fine-tune protein activity by reversible modification rather than altering genetic information.
This makes post-translational control a crucial level of gene expression regulation complementing transcriptional and translational controls.
Summary of Key Terms and Molecules
cAMP – secondary messenger triggering phosphorylation cascades.
Adenylate cyclase – enzyme converting ATP to cAMP.
Protein kinase A (PKA) – enzyme activated by cAMP to phosphorylate target proteins.
Phosphorylation – process activating or deactivating proteins through phosphate addition.
Phosphodiesterase – enzyme that degrades cAMP, switching off the signal.
Through these mechanisms, cells efficiently activate proteins after translation, aligning perfectly with the OCR specification’s emphasis on phosphorylation cascades triggered by cyclic AMP.
FAQ
Phosphorylation rapidly changes the shape and charge of a protein, altering its activity almost instantly without requiring new protein synthesis.
This modification can:
Activate or deactivate enzymes by altering their active site.
Enable protein–protein interactions or cause proteins to dissociate.
Be reversible, allowing tight control through phosphatases that remove phosphate groups.
Because it requires little energy and offers flexibility, phosphorylation allows cells to adjust metabolic or signalling pathways within seconds of receiving a stimulus.
cAMP is degraded by the enzyme phosphodiesterase (PDE), which converts it into AMP, a molecule that no longer activates protein kinase A (PKA).
In addition:
Phosphatases remove phosphate groups added during the cascade, reversing activation.
Receptors and G proteins are reset as the hormone signal stops.
This ensures that enzyme activation is temporary and controlled, preventing unnecessary energy expenditure or prolonged stimulation.
Secondary messengers amplify weak external signals into large intracellular responses.
For example:
One hormone molecule activates one GPCR, producing many cAMP molecules.
Each cAMP activates multiple PKA molecules, which phosphorylate many downstream targets.
This amplification ensures sensitivity and speed, while maintaining specificity — different cells can interpret the same cAMP signal differently depending on which kinases and proteins they express.
Phosphorylation can both activate and inhibit proteins, depending on where the phosphate group is added and the structure of the protein.
In glycogen metabolism, phosphorylation activates glycogen phosphorylase but inhibits glycogen synthase.
Structural changes can close or open the active site or alter the protein’s binding affinity.
This dual role allows cells to coordinate opposing processes, ensuring metabolic balance during responses like stress or exercise.
cAMP is diffusible, stable, and versatile, making it ideal for coordinating widespread responses.
Advantages include:
It can easily move through the cytoplasm, enabling signal propagation across the cell.
It’s generated and removed enzymatically, allowing precise control of concentration.
It operates independently of ion gradients, unlike calcium, reducing cross-talk with other pathways.
These traits make cAMP a key messenger in hormonal regulation and metabolic control, especially in vertebrate cells.
Practice Questions
Question 1 (2 marks)
Explain how cyclic AMP (cAMP) can cause the activation of protein kinase A (PKA) in cells.
Mark scheme:
1 mark: cAMP binds to the regulatory subunits of inactive PKA.
1 mark: This causes the release of catalytic subunits, which become active and can phosphorylate target proteins.
Question 2 (5 marks)
Describe how a rise in cyclic AMP (cAMP) concentration can lead to the activation of enzymes involved in glycogen breakdown. Include in your answer the key enzymes and the role of phosphorylation in this process.
Mark scheme:
1 mark: Adrenaline or another hormone binds to a G protein-coupled receptor (GPCR), activating the G protein.
1 mark: The G protein activates adenylate cyclase, which converts ATP to cAMP.
1 mark: cAMP activates protein kinase A (PKA) by binding to its regulatory subunits and releasing active catalytic subunits.
1 mark: Active PKA phosphorylates glycogen phosphorylase kinase, activating it.
1 mark: Glycogen phosphorylase kinase then phosphorylates glycogen phosphorylase, which breaks down glycogen to glucose-1-phosphate for respiration.
Alternative phrasing of enzyme names or sequence accepted if correct and logically explained.

