OCR Specification focus:
‘Measure gradients and intercepts accurately from linearised plots to determine relationships and constants from experimental data.’
Understanding Gradients and Intercepts
In A-Level Chemistry, graphs are powerful tools for analysing experimental data and determining relationships between variables. By carefully measuring gradients and intercepts, chemists can extract meaningful quantities such as rate constants, equilibrium constants, and enthalpy changes. Mastering these skills ensures accurate interpretation of results and supports valid conclusions based on evidence.
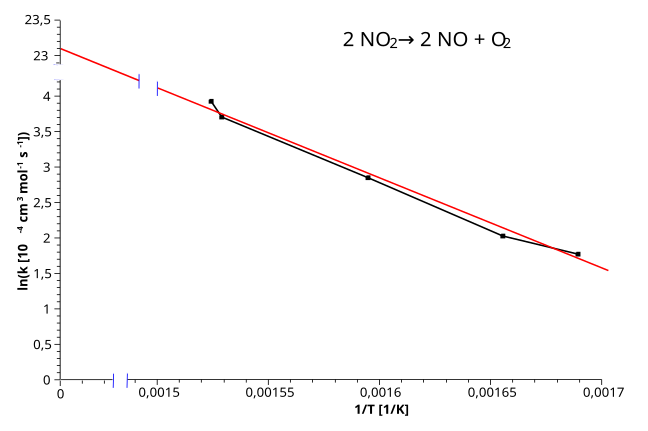
Arrhenius plot showing ln k versus 1/T with a clear straight-line fit. The gradient (−Ea/R) and y-intercept (ln A) can be read directly to obtain kinetic parameters. The axis break highlights the intercept region for clarity. Source
Importance of Linear Relationships
Many chemical relationships can be expressed as linear equations, which take the general form:
Straight-line Equation (y = mx + c)
y = Dependent variable (measured quantity)
x = Independent variable (controlled quantity)
m = Gradient (rate of change between variables)
c = Intercept (value of y when x = 0)
Linearising non-linear relationships allows experimental data to be interpreted more easily. For example, in rate or thermodynamic studies, transforming equations to a linear form enables determination of constants through graphical methods.
Selecting Appropriate Axes
Choosing suitable axes is crucial to ensure accurate measurement of gradients and intercepts.
The independent variable should be plotted on the x-axis, as it is the quantity the experimenter controls.
The dependent variable should be plotted on the y-axis, as it changes in response.
Both axes must include units and consistent scaling to maintain proportional spacing between data points.
Where relationships involve logarithmic or reciprocal values, appropriate transformations (e.g., plotting 1/[A] or ln p) should be made before graphing.
Proper axis selection ensures that the resulting plot correctly represents the underlying mathematical relationship.
Measuring the Gradient
The gradient represents the rate of change of one variable with respect to another. It quantifies how much the dependent variable changes for each unit change in the independent variable.
Gradient: The change in the dependent variable (Δy) divided by the corresponding change in the independent variable (Δx).
Gradient (m) = Δy / Δx
Δy = Difference between two y-values (units of dependent variable)
Δx = Difference between two x-values (units of independent variable)
To obtain an accurate gradient:
Draw the line of best fit that represents the overall trend of the data.
Mark two well-separated points on the line (not necessarily data points).
Read coordinates accurately, including units and decimal precision.
Calculate Δy and Δx, ensuring consistent units.
Large, well-separated points minimise percentage error and improve reliability of the calculated gradient.
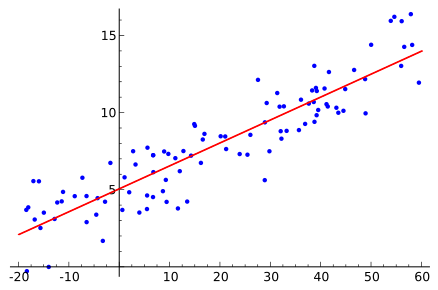
Scatter plot with a straight regression line. The gradient (rise over run) is taken from any convenient triangle on the line, and the intercept is the point where the line crosses the y-axis. Axes are clearly labelled to emphasise units. Source
Determining the Intercept
The intercept is the value of the dependent variable when the independent variable equals zero. It represents the point where the line crosses the y-axis.
Intercept: The value of y when x = 0, representing the constant term (c) in a linear relationship.
In experimental chemistry, intercepts can reveal important constants:
In Arrhenius plots (ln k vs 1/T), the intercept corresponds to ln A, the natural logarithm of the pre-exponential factor.
In Beer–Lambert law graphs (A vs c), the intercept ideally equals zero if no systematic error or instrument offset exists.
In calibration curves, the intercept indicates any baseline offset or background signal.
Accurate determination of the intercept allows meaningful comparisons between theoretical and experimental constants.
Using Graphical Methods to Determine Constants
Experimental data are often transformed into linear forms to allow constants to be derived from the gradient or intercept. Examples include:
Rate equations: For first-order reactions, plotting ln [A] against time gives a straight line with gradient = −k.
Arrhenius equation: Plotting ln k against 1/T yields a gradient = −Ea/R and intercept = ln A.
Gas laws: A plot of P versus T (for constant volume) gives gradient = nR/V.
In each case, accurate graphical analysis ensures correct determination of key physical or chemical parameters.
Ensuring Accuracy and Precision in Measurement
To achieve reliable graphical results:
Always use axes that cover at least two-thirds of the available graph paper to reduce reading uncertainty.
Label axes clearly with quantities and units.
Avoid joining data points directly; instead, draw a best-fit line using a ruler or software.
When estimating the gradient, use a large triangle between two points on the line to reduce rounding and measurement error.
Maintain consistent significant figures that reflect the precision of the experimental data.
Accurate plotting and measurement contribute directly to the validity and reproducibility of analytical results.
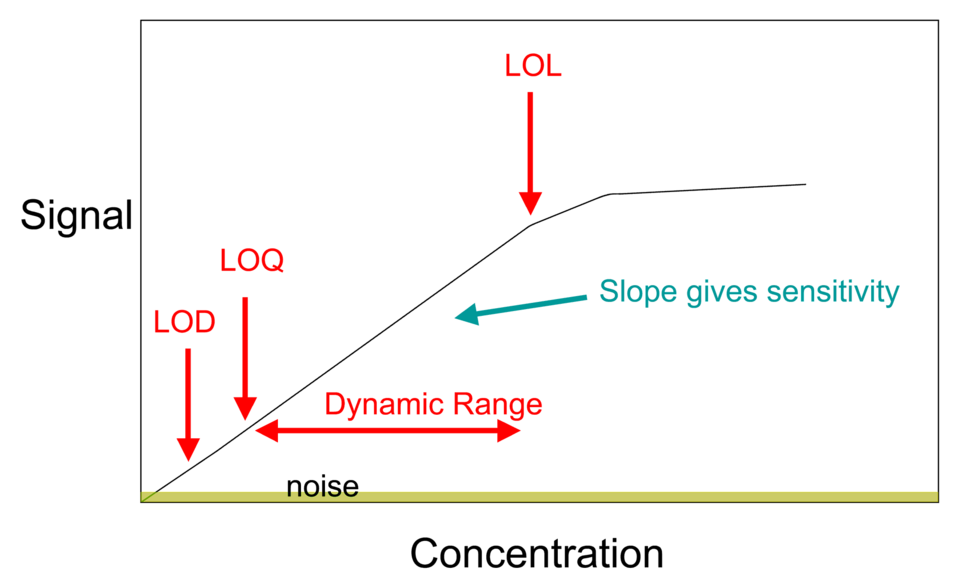
Calibration curve with a straight-line fit suitable for obtaining gradient and y-intercept from analytical data. This version additionally labels LOD, LOQ, and linearity limits; these features exceed the syllabus scope but the straight-line elements remain directly applicable. Source
Recognising and Reducing Errors
Common sources of error when measuring gradients and intercepts include:
Poorly scaled axes, leading to distorted slopes.
Incorrectly drawn best-fit lines, especially if biased towards certain data points.
Inconsistent unit conversions, causing gradient misinterpretation.
Limited data spread, which increases uncertainty in slope measurement.
Graph reading errors, such as parallax or rounding mistakes.
Reducing these errors involves careful plotting, consistent units, and repeated analysis. Modern data software can also be used to minimise human error while retaining understanding of manual methods.
Using Linearised Plots Effectively
Not all relationships are naturally linear. In such cases, linearisation helps simplify complex expressions. For instance:
The Arrhenius equation, k = A e^(−Ea/RT), becomes ln k = −Ea/R (1/T) + ln A when linearised.
The first-order rate law, [A] = [A]₀ e^(−kt), becomes ln [A] = −kt + ln [A]₀.
By transforming these relationships into straight-line form, chemists can determine constants like activation energy (Ea) or rate constant (k) from the gradient and intercept of the plot.
Recording and Reporting Graphical Results
After measuring the gradient and intercept:
Express the gradient with appropriate units derived from the variables’ quantities (e.g., mol dm⁻³ s⁻¹ per K).
Record the intercept value with matching significant figures to ensure consistency.
Clearly annotate graphs with any lines used to determine Δx and Δy.
Include a short note describing how constants were obtained and what they represent chemically.
Proper recording practices ensure clarity in data analysis and support transparent evaluation during assessment.
Application in Experimental Evaluation
Evaluating the accuracy of a graph involves checking whether the gradient and intercept align with expected theoretical values. Deviations may indicate:
Measurement or systematic errors.
Incorrect unit conversions.
Experimental design flaws affecting precision.
Through accurate determination of gradients and intercepts, students demonstrate their ability to link quantitative data to chemical principles, fulfilling the OCR requirement to “measure gradients and intercepts accurately from linearised plots to determine relationships and constants from experimental data.”
FAQ
Gradients and intercepts are frequently used in kinetic and thermodynamic studies. Examples include:
Arrhenius plots (ln k vs 1/T) to find activation energy and pre-exponential factors.
First-order reaction plots (ln [A] vs time) to determine rate constants.
Beer–Lambert law graphs (A vs c) for calibration in spectroscopic analysis.
Gas law graphs (P vs T or V vs 1/P) for determining constants like R.
These allow quantitative interpretation of data through linear relationships.
Selecting widely separated points reduces the impact of small reading or rounding errors.
A small triangle gives a large percentage uncertainty because minor changes in Δx or Δy significantly affect the ratio.
Using a large, well-spaced triangle:
Minimises error propagation.
Improves accuracy of the gradient value.
Ensures that random fluctuations in individual data points have less influence on the slope.
Uncertainty is estimated by drawing maximum and minimum gradient lines that still fit within the data’s scatter.
The maximum gradient line passes through the highest plausible points.
The minimum gradient line passes through the lowest plausible points.
The difference between these gradients gives a measure of uncertainty:
Uncertainty = (max gradient – min gradient) / 2.
This provides a visual method for assessing reliability in experimental data interpretation.
Even in a linear relationship, data scatter can occur due to:
Measurement uncertainty in apparatus readings.
Fluctuating conditions, such as small temperature variations.
Instrument calibration errors or background interference.
As a result, a line of best fit is drawn to represent the average trend of the data, rather than connecting every point exactly.
Conventionally, the independent variable (controlled by the experimenter) is placed on the x-axis, and the dependent variable (measured response) is placed on the y-axis.
This arrangement ensures that the gradient represents how the dependent variable changes in response to changes in the independent variable.
For transformed or linearised relationships, always check the rearranged equation to identify which variable corresponds to x and y before plotting.
Practice Questions
A student plots a graph of ln k (y-axis) against 1/T (x-axis) for a chemical reaction.
(a) State what the gradient and intercept of this line represent.
(2 marks)
1 mark: Gradient represents –Ea/R, where Ea is the activation energy.
1 mark: Intercept represents ln A, the natural logarithm of the pre-exponential factor.
An experiment to determine the rate constant, k, of a reaction is carried out at several temperatures. The student plots ln k against 1/T and obtains a straight line.
(a) Explain how the gradient and intercept can be used to calculate the activation energy and the pre-exponential factor. (3 marks)
(b) Suggest two reasons why the experimental points might not lie exactly on the straight line. (2 marks)
(5 marks)
(a)
1 mark: The equation ln k = –Ea/R (1/T) + ln A relates ln k and 1/T.
1 mark: Gradient = –Ea/R, so Ea = –gradient × R.
1 mark: Intercept = ln A, so A = e^(intercept).
(b)
1 mark: Random errors in temperature measurement or rate determination.
1 mark: Systematic errors such as instrumental drift or impurities affecting reaction rate.

